Respiratory Researchers Share Findings at International Thoracic Conference
Published
Featured Researchers
Researchers from Seattle Children’s Research Institute shared findings to more efficiently treat, cure and diagnose asthma, cystic fibrosis, bronchopulmonary dysplasia and more at the annual American Thoracic Society (ATS) International Conference, held May 18 to May 21 in San Francisco.
Thirty-three oral and poster abstracts were presented by 24 researchers from the Center for Respiratory Biology and Therapeutics (CRBT), alongside additional Children’s researchers. Each year, nearly 14,000 researchers from around the world attend the conference to learn about the latest advances in respiratory research, interact with international colleagues and kindle new collaborations. Seattle Children’s stood out as a leader in the field, highlighting the depth, innovation and impact of its respiratory research program on a global stage.
“The ATS conference is the biggest and one of the most important meetings where researchers and clinicians across pulmonary, critical care and sleep medicine present their work on a global stage,” said CRBT Director Jason Debley, MD, MPH. “It serves as the premier platform for unveiling cutting-edge respiratory research from basic through translational, clinical, epidemiologic, community and global health and brings together physicians, basic researchers, nurses, respiratory therapists and industry leaders, fostering cross-disciplinary dialogue and partnerships.”
Seattle Children’s, and the Center for Respiratory Biology and Therapeutics in particular, has a large presence at ATS annually, with many CRBT faculty across several Seattle Children’s clinical divisions selected each year to present their work in prestigious scientific ATS symposia.
— Jason Debley, MD, MPH, Director, Center for Respiratory Biology and Therapeutics
The Debley Lab researches the role airway epithelial cells (cells lining the airway) play in asthma, health and lung development. Dr. Debley, his lab team and faculty mentees presented at this year’s ATS.

One of Dr. Debley’s symposium talks, sponsored by NIH, shared results from a study that explored why children with exacerbation-prone asthma are more vulnerable to infection with rhinovirus, the virus responsible for the common cold. The study found that airway epithelial cells from these children permit excessive replication of the virus due to weaker baseline immune activity which then leads to excessive airway inflammation following infection.
In this work, the Debley lab observed that treating airway cells from exacerbation-prone children with a specific immune-boosting protein (Interferon-β) markedly reduced replication of rhinovirus and calmed secondary exaggerated immune and inflammatory responses, suggesting a possible way to prevent asthma attacks triggered by rhinovirus infections. This work not only sets the stage for future trials to prevent exacerbations and improve baseline lung function in exacerbation-prone children, but also identifies novel therapeutic targets.
“I believe that by learning from patients and applying cutting-edge research tools and techniques to human cell and tissue model systems from children with and without asthma, we can greatly advance our understanding of how airway epithelial cells respond to viral infections and environmental factors (e.g. smoke, pollens, etc.), leading to airway inflammation and injury,” Dr. Debley said. “By understanding these mechanisms, we can develop better treatments and hopefully, a cure for asthma someday.”
Oral abstracts presented by CRBT researchers also include:

From Cradle to Chronic Disease: Diagnosis and Timely Treatment
“Premature birth accounts for 10% of live births and disrupts normal airway development. Adult survivors of premature birth develop lifelong airway disease and early-onset chronic obstructive pulmonary disease. Yet, we lack targeted therapies to achieve and sustain normal lung function. Our research has identified that cells that line the airways of premature infants make more of a protein called vimentin; this protein may change the structure of the airway and cause airway obstruction. We hope this discovery will enable earlier identification of airway disease in premature infants and guide future development of targeted therapies.”
— Laurie Eldredge, MD, PhD, principal investigator, and Yan Han, research scientist, Eldredge Lab

Safer, Non-Surgical Ways to Improve Lung Function Over Time
“My research crosses the fields of early onset scoliosis in young children and quantitative respiratory assessments in pediatrics. I work with spine surgeons internationally to develop new surgical and non-surgical ways to maximize lung function over time. This includes measuring functional outcomes as spine deformities progress and as they are treated.”
— Greg Redding, MD, principal investigator, Pulmonary and Sleep Medicine

Exploring Asthma at the Cellular Level
“DNA methylation is one of the ways that our cells integrate signals from the environment and experiences to change how they function and turn genes on and off. We looked at how DNA methylation changes in airway cells after infection with rhinovirus or exposure to the cytokine IL-13 (which is increased with allergic inflammation) and found that both lead to changes at multiple sites in the genome and change gene expression. Future work is planned to look at how these changes in cells explain why children are more prone to asthma and to asthma exacerbations triggered by viral infections.”
— Weston Powell, MD, PhD, principal investigator, Powell Lab / Debley Lab

Wider and Better Outcomes for Cystic Fibrosis
“I had a young girl with cystic fibrosis who had such severe lung disease that she couldn’t attend school and couldn’t sleep at night due to her cough. She inspired me to research what is causing some patients to have very severe lung disease despite getting lots of medications and therapies. Our current research has found that Latino children with cystic fibrosis and CFTR-related metabolic syndrome (CRMS) had less healthy bacteria, more pathogenic bacteria, and increased inflammation in their airways compared to non-Latino children.”
— Meghan McGarry, MD, MAS, principal investigator, McGarry Lab
Empowering the Next Generation of Research Leaders
Terri Laguna, MD, MSc, division head of pulmonary and sleep medicine at Seattle Children’s and cystic fibrosis researcher at the Laguna Lab presented research and was the featured speaker at one of the conference’s forums, encouraging authentic and representative scientific leaders in research.
Seattle Children’s Researchers Present Posters and Talks at ATS Conference
Several other scientists and physician-researchers from Seattle Children’s contributed the following posters and presentations at ATS:
Jason Debley, MD, MPH
- IL-33 Modulates Crosstalk Between Eosinophils and Airway Epithelial Cells
- Airway Epithelial Responses to Human Rhinovirus A16 Infection Are Differentially Regulated by the Presence of Monocytes
- Mast Cells Modulate the Transcriptome of Epithelial Subpopulations in Model of the Airway Epithelial Microenvironment
- Gaining Mechanistic Insights, Designing Novel Therapeutics, and Engineering Precision Delivery in Pediatric Lung Diseases
Jessica Clarion, MD, pediatric pulmonary fellow
Mary Crocker, MD, MPH, principal investigator, Crocker Research Program
Sydney Clark, research scientist, Reeves Lab
Camille Gates, research technician, Debley Lab
Gail Deutsch, MD, associate CRBT center director, pathologist
Matt Fought, research scientist, Laguna Lab
- Inflammation Unleashed: Uncovering Relationships Between Cathepsin B and Common Cystic Fibrosis Pathogens
- Molecular Methods Versus Culture to Detect and Quantify Fungal Communities in Airway Specimens
Courtney Gallagher, MD, physician
Blair Mockler, MD, pediatric pulmonary fellow
- Antibiotic Treatment Following Bronchoscopy with Bronchoalveolar Lavage Among Children with Chronic Cough
- From Breathlessness to Diagnosis: High Altitude Reveals Pulmonary AVMs and Diagnosis of Hereditary Hemorrhagic Telangiectasia
- A Case of Acute Obstructive Pulmonary Physiology due to Stevens Johnson Syndrome/Toxic Epidermic Necrolysis
Laura Ellington, MD, MS, principal investigator, Ellington Lab
- Association of Quantitative CT Airway Metrics with Impaired Lung Function Among Adolescents with HIV in Nairobi, Kenya
- User-centered Design of a Digital Clinical Decision Support Tool to Aid in the Diagnosis and Management of Outpatient Pediatric Respiratory Illnesses in Uganda
Weston Powell, MD, PhD, Powell Lab
- Circadian Regulation of Viral Infections and Inflammation in Pediatric Asthma
- Priming of Human Bronchial Epithelial Cells Enhances Viral Replication and Interferon Signaling
Rebecca Spurr, MD, pediatric pulmonary fellow
MacKenzie Wyatt, MD, pediatric pulmonary fellow
- When Breathing Becomes Botched: Infant Botulism Leading to Respiratory Failure
- Collateral Damage: Hemoptysis in a Child with Fontan Physiology
- Rapid Development of Plastic Bronchitis in a Child with a Fenestrated Fontan
- CFTR-IFIC Reclassification: When Cystic Fibrosis Transmembrane Conductance Regulator-Related Metabolic Syndrome (CRMS) Gets a Makeover!
- A Fluid Relationship: Superior Vena Cava Syndrome and Chylothorax in Child with Sickle Cell Disease
Ben Wilfond, MD, principal investigator, Wilfond Lab
- The Ethical Importance of Community Partnerships: Challenges and Opportunities
Elizabeth Vanderwall, research scientist, Debley Lab
Research Progress Toward Safer Hemophilia A Treatments
Three poster presentations from research scientists on the Miao Lab team show progress toward safer, more effective treatments for hemophilia A, a genetic disorder where the blood doesn't clot properly due to lack of a protein called factor VIII (FVIII).
Chun-Yu Chen and colleagues developed a novel CRISPR/Cas9-based strategy to repair the missing exon of the FVIII gene in a preclinical model of hemophilia A. This gene repair would allow patients to make the protein needed to stop bleeding and help wounds heal properly.
Meng-Ni Fan and team are experimenting with a lipid nanoparticle-based non-viral delivery of FVIII to treat the blood-clotting disorder. The treatment lasted for many months and could be given again safely, offering hope for a new type of gene therapy. The research could pave the way for safer and repeatedly administered gene therapies, making these treatments more accessible for various genetic disorders that require long-term intervention.
Ting-Yen (Scott) Chao and collaborators are asking: Can ultrasound-mediated gene delivery effectively and safely treat hemophilia A by fixing the faulty gene? They used ultrasound and microbubbles to deliver CRISPR/Cas9 gene-editing tools in preclinical models, fixing the faulty gene and restoring some clotting ability.
“As an abstract reviewer for ASGCT, I can attest that the high acceptance rate of our abstracts indicates the high quality and excellence of the scientific advances from Children’s researchers,” said principal investigator Carol Miao, PhD, who is co-chairing two oral abstract sessions and a scientific symposium at this year’s meeting.
Cell Therapy Advances in Solid Tumor Therapies, Immune System Reprogramming, and B Cell Biology
Other Children’s poster presenters share research insights into creating better solid tumor therapies, reprogramming the immune system to halt autoimmune diseases, and better understanding B cell biology.
Miranda Lyons-Cohen, PhD, an Invent at Seattle Children’s postdoctoral scholar, and graduate student Simonne Guenette, both in the Ben Towne Center’s Oda Lab, are each presenting posters on development of next-generation cell therapies to treat pediatric cancers. They and their team are engineering strategies that enable T cells to overcome obstacles in the tumor microenvironment and mount a more powerful, durable and sustained attack on cancer cells by replacing a T cell brake with an accelerator.
To treat disorders involving pathogenic antibodies, including lupus and organ transplant rejection, Peter Cook, a research scientist in the Rawlings Lab, and colleagues have developed a new type of cell-based medicine that can target and kill the plasma cells that secrete problematic antibodies. Such an approach could stop the immune system from attacking healthy tissues in autoimmune diseases like type 1 diabetes and multiple sclerosis and reduce the chance of rejection in solid organ transplantation.
“ASGCT offers the opportunity to see the broader scope of the field and get a sense of different technologies that are being developed within the cell and gene therapy space,” Cook said. “We can see the real benefit these therapies are providing to patients in the form of clinical trial data that is frequently presented at the meeting.”
Nikita Trivedi, a research scientist in the Center for Immunity and Immunotherapies’ James Lab, analyzed the role of integrins — proteins that facilitate cell-to-cell communication — in antibody-producing human plasma cells in a preclinical model, revealing that two key integrins are partially required for plasma cells to localize to the bone marrow, challenging previous assumptions. However, enhancing their expression with retinoic acid directed plasma cells to abdominal fat, offering potential for site-specific B cell therapeutic delivery.
Principal investigator Rich James, PhD, is excited for Nikita and others to share their findings at ASGCT. “Our team members will get specific feedback on their presentations from experts from a diverse set of fields/perspectives, and this helps guide and prioritize future studies,” he said.
“At meetings like ASGCT, we are exposed to the newest and most exciting ideas in gene therapy, which we hope they can bring back to the lab for implementation,” Dr. James said.
Related Categories
Featured Researchers
Related Centers & Programs
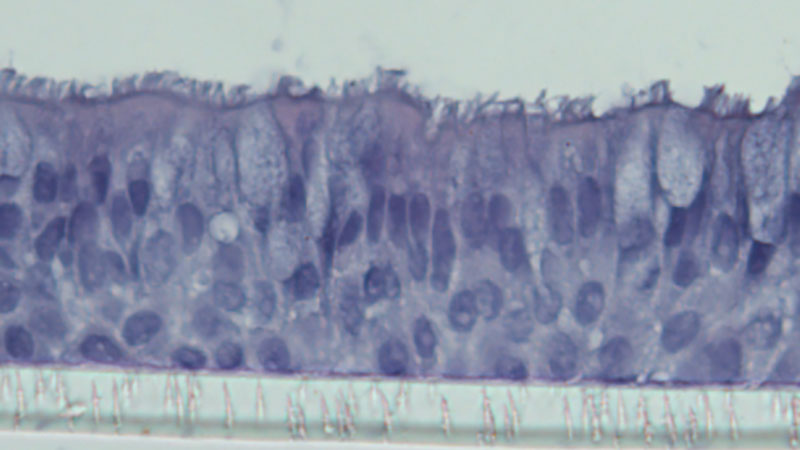
Airway Cells in Children With Allergic Asthma Protect Against COVID-19 Infection
Published
Seattle Children’s researchers and colleagues discovered why kids with allergic asthma fare better against COVID-19.
A Fresh Breath For Respiratory Research
Published
A center at Seattle Children’s Research Institute aims to pioneer treatments, advance technologies and refine surgical approaches for respiratory conditions.

Seattle Children’s and WSU Host Inaugural Joint Research Symposium
Published
Collaboration between Seattle Children’s and WSU Health Sciences catalyzes new research opportunities.

