Adjustable Pediatric Heart Stent Moves to Pivotal Trial
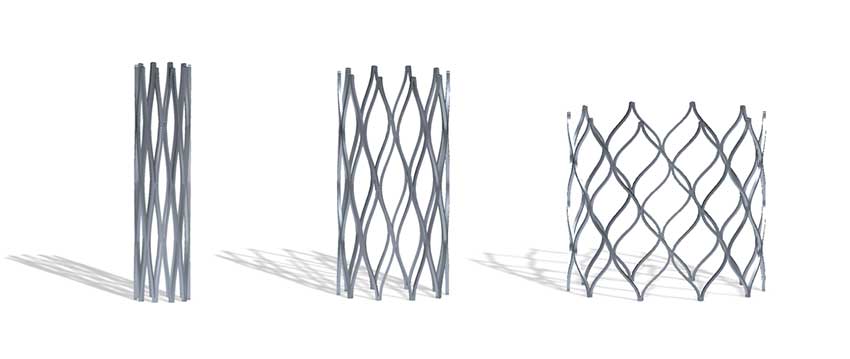
The Renata Minima Stent can be gradually expanded up to adult size over the course of the child’s lifetime.
Infants and children with congenital heart disease are one step closer to a lifelong solution, thanks in part to Seattle Children’s Heart Center team.
In spring, 2022, Seattle Children’s was one of only four pediatric hospitals in the nation to participate in an early feasibility study of a new minimally invasive cardiac stent developed to treat narrowing of the pulmonary arteries or the aorta. Designed to expand as younger patients grow, the Renata Minima Stent holds the promise of reducing the need for repeated risky and expensive open-heart surgeries to replace stents that have become too small.
According to Dr. Brian Morray, Director of Cardiac Catheterization Labs at Seattle Children’s, “there’s a huge need for this technology. There are currently no FDA approved stents for use in pediatrics. “
Pediatric stents present challenges not found in adults–namely that adults will not outgrow their stents. With young children, and infants in particular, stents become ineffective as their arteries grow, requiring them to be redilated or replaced at multiple points during their lives.
“Developing a stent that’s small enough for an infant but can accommodate for growth is an engineering challenge that most companies have not been willing to take on,” said Morray.
 Dr. Brian Morray is Director of Cardiac Catheterization Labs at Seattle Children’s
Dr. Brian Morray is Director of Cardiac Catheterization Labs at Seattle Children’s“The fact that this stent is designed specifically for small children and infants is very unique. It is rare that a company would have any interest in developing something specifically for this small and vulnerable population.”
The first patient at Seattle Children’s to receive the Renata stent was a child under the age of two who had it implanted in their pulmonary artery. Previously, the child would have received a non-pediatric stent, which would have required redilation multiple times and ultimately intentional fracture or replacement by the time they reached adolescence. But because Seattle Children’s was one of only four sites in the United States enrolled in this feasibility study, they were able to receive the Renata stent which can be expanded up to adult size and accommodate for the patient’s growth.
Over the course of two months, Renata stents were successfully implanted in nine other pediatric patients throughout the four study sites. In fall, 2022, the Renata stent progressed to the next phase towards FDA approval, known as a pivotal trial.
The pivotal trial is also called an IDE trial by the FDA. IDE stands for investigational device exemption and is a regulatory pathway created by the FDA that provides the pathway for approval for a medical device. In this phase of the FDA approval process, Seattle Children’s and other participating medical centers enroll additional patients and follow them for a longer period of time.
Seattle Children’s patients are followed by the medical center’s research team in collaboration with Renata Medical. The data collected from both the early feasibility study, as well as the IDE portion of the study, will all be sent to the FDA for approval.
The FDA granted approval to expand the pivotal trial to 6 participating sites, two more than were included in the early feasibility study. According to Morray, “the final enrollment number will likely be 40-50 patients and Seattle Children’s will enroll as many patients as we have and can.”
Doctors at Seattle Children’s performed the first Minima stent implantation during the pivotal trial in October 2022 and are currently scheduling additional patients for participation in this important study. So far, three patients have undergone the procedure at Seattle Children’s and two more are set to be scheduled in the near future.
The early feasibility study completed enrollment within two months and the next phase is moving quickly as well.. “During the early feasibility study, the four sites had patients waiting that were eligible but couldn't get in,” said Morray. “There is a lot of excitement surrounding this device and we are really pleased to be able to offer this new technology to our patients.”
Contact Seattle Children’s Heart Center at 206-987-2015 for a referral, a second opinion or more information.