Publication Q&A: Reduced Function of the Adaptor SH2B3 Promotes T1D via Altered Cytokine-Regulated, T cell Intrinsic Immune Tolerance
Published
Featured Researchers
Type 1 diabetes (T1D) is one of the most common chronic diseases affecting children in the U.S. Over 1 million people live with T1D, and that number is expected to grow to 5 million by 2050.
T1D is a lifelong, progressive disease caused by an autoimmune reaction. While it usually develops in children or young adults, anyone at any age can develop T1D. Some, including those with a family history of T1D or those who have other autoimmune conditions, are at higher risk.
In T1D, the immune system mistakenly attacks and damages healthy insulin-producing cells called beta cells. Insulin helps convert food to energy in the body. In the later stages of T1D, the pancreas doesn’t make any insulin. Without insulin, blood sugar levels become too high, which can lead to life-threatening complications.
Historically, the majority of patients with T1D have been diagnosed at stage 3 of disease development, after significant beta-cell destruction and the presence of other clinical symptoms. Researchers are working to define genetic risk variants to identify high-risk individuals or predict T1D progression, allowing for earlier intervention to preserve beta-cell mass. Scientists hope to better understand the preclinical phases of T1D and find new predictive biomarkers for earlier diagnosis and therapy, with the final goal of reverting or even preventing the development of the disease.
The genetic region near the SH2B3 gene has been strongly linked to risk for developing T1D, leading Seattle Children’s Research Institute’s Center for Immunity and Immunotherapies’ researchers to directly test the role of this regulatory protein in a preclinical model of T1D.

(Left to right) Taylor Watson, Dr. Eric Allenspach, Dr. David Rawlings
Led by Taylor Watson, a research scientist in the Allenspach Lab, under the direction of Eric Allenspach, MD, PhD, and David Rawlings, MD, the collaborators observed that decreased SH2B3 function resulted in enhanced survival of autoreactive cells against the pancreas and accelerated T1D development.
Children’s contributing authors: Taylor Watson (first author), Travis Drow, Jacob Medjo, Matthew MacQuivey, David Rawlings, MD (co-corresponding author) and Eric Allenspach, MD, PhD (co-corresponding author).
This research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
Read this article in the journal Diabetes (published online March 6, 2025, ahead of print).
How can this immunology research lead to better ways to treat or prevent Type 1 diabetes?
A genetic variant in the SH2B3 gene results in a protein change that reduces the functionality of this critical regulatory protein that has been credibly associated with increased risk for T1D. This is a common genetic variant in populations of European descent and has been now linked to therapeutic outcomes in clinical trials of patients with T1D.
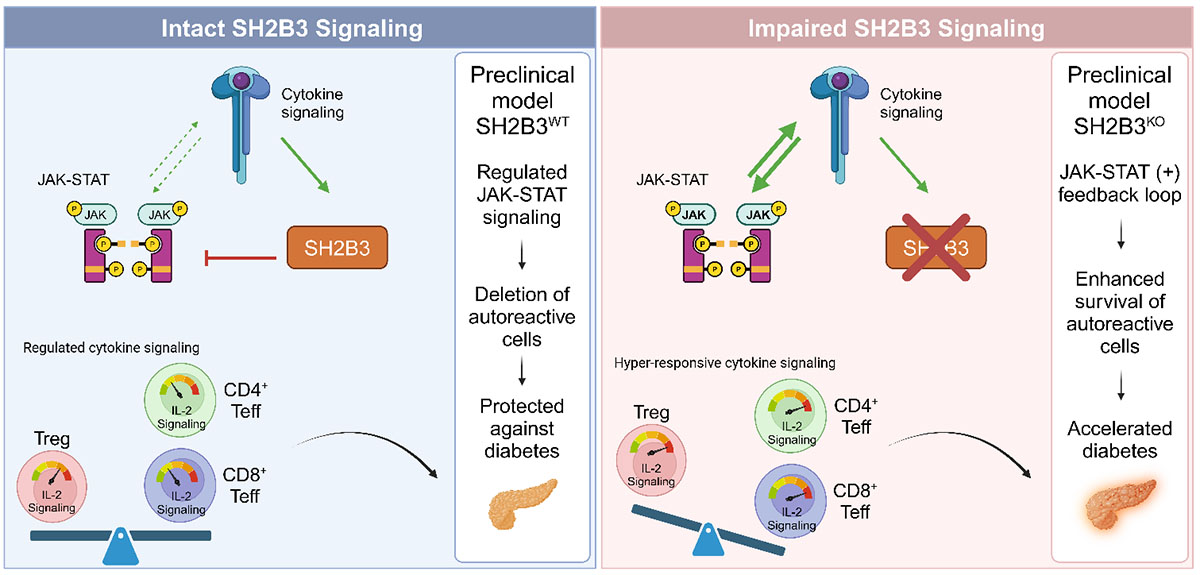
In this paper, we characterize potential mechanisms through which SH2B3 deficiency contributes to cytokine hypersensitivity, enhanced autoreactive T cell survival and subsequent T1D development. This is of interest clinically, as recent trends in T1D treatment have focused on identifying and treating patients in the early stages of disease, prior to significant beta-cell loss and insulin dependency. Characterizing the role of SH2B3 in T cell signaling can better inform the treatment of at-risk patients with decreased SH2B3 function.
Is this Type 1 diabetes research a first in any way?
Yes, we are the first to describe how loss of the SH2B3 gene contributes to T1D development specifically through T cells.
What are the next steps and long-term goals for this T cell research?
We are currently creating a preclinical research model in which the SH2B3 gene will be knocked out only in T cells, allowing us to better test whether reduced function of SH2B3 in T cells alone is sufficient to cause accelerated diabetes. Given that T1D is a polygenic disease — multiple genetic risk factors contribute to the disease — we want to use this preclinical model to identify other genetic risk factors that encode proteins that interact with SH2B3 either directly or functionally.



