Jaspan Lab

The Jaspan Lab seeks to better understand the interplay between host microbial and immunological factors that impacts susceptibility to infectious diseases in infant and adolescents from sub-Saharan Africa. Our overarching goal is to pinpoint mechanisms by which dynamic community structures of commensal bacteria regulate immunology at mucosal surfaces and how that ultimately mitigates or promotes susceptibility to infectious disease. We additionally seek to elucidate the role that maternal HIV infection has on these commensal communities, and the resultant effects of HIV exposure on infant immunity immunology and vaccine responses. We utilize clinical, preclinical and computational approaches to address these topics.
Altered Microbiota and Immunity of HIV-Exposed Infants
Infants who are HIV-exposed but remain uninfected (iHEU) experience higher rates of infectious morbidity and mortality compared to infants who are HIV-unexposed uninfected (iHUU). iHEU display reduced vaccine responses, which likely contributes to their increased disease. The development of certain T cell subsets, both in the mucosa and systemically, is determined by the presence of specific microbes in the gut and may be important in determining adaptive immunity and lasting vaccine responses. As the gut microbiota of iHEU differs from that of iHUU, our working hypothesis is that altered gut microbiota in iHEU influence infant immunity and subsequent vaccine responses, which ultimately may contribute to enhanced disease susceptibility.
We additionally aim to understand how maternal factors, including breastmilk/chestmilk and the expanded enteric virome of mothers living with HIV affects the development of the bacterial and viral consortia and immune response of related infants. We are employing a top-down approach by integrating bacterial community surveys with viral metagenomics and flow cytometry based measurement of T-cell responses to BCG vaccination. Insights from statistical analysis of our clinical cohort data will guide downstream experiments assaying the effect of specific viral taxa on the bacterial community and BCG-specific response in gnotobiotic animals. Findings from these studies could lead to interventions such as pre-or probiotics to improve immunity in HIV-exposed infants.
Finally, in research studies of healthy infants, the bacille Calmette-Guérin (BCG) vaccine given at birth has been found to induce benefits that extend beyond protection from tuberculosis (TB) meningitis and disseminated TB, and provides non-specific protection against other bacterial infections during the first years of life. To identify if and how HIV exposure alters benefits conferred by the BCG vaccine, we are evaluating BCG vaccine-induced TB specific and non-specific immune responses among iHEU compared to iHUU. Specifically, we aim to identify the extent of signature epigenetic changes and “trained innate immunity” in these infants. We further aim to determine if those changes impact functional parameters of innate cells, like monocytes and NK cells.
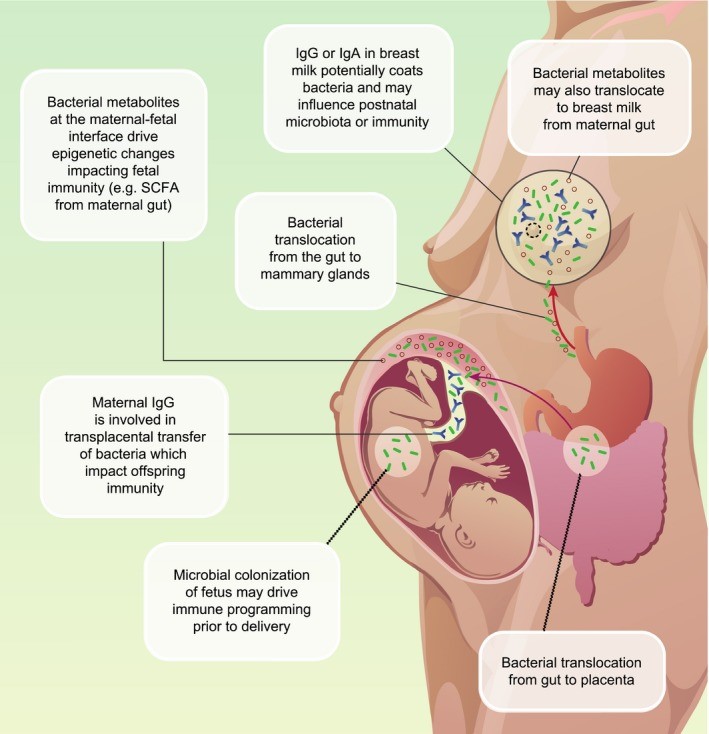
Nyangahu DD, Jaspan HB. Influence of maternal microbiota during pregnancy on infant immunity. Clin Exp Immunol. 2019 Oct;198(1):47-56. doi: 10.1111/cei.13331. Epub 2019 Jun 21. PMID: 31121057; PMCID: PMC6718277.
Genital Tract Microbiota and Infectious Disease Susceptibility
The genital microbiota can be modulated by physiological factors such as contraceptive choices or endogenous hormones. In turn, microbial changes occurring in the genital tract can influence a woman’s mucosal immune responses and susceptibility to sexually transmitted infections (STIs) or yeast infections. Using an array of sequencing techniques, our lab investigates how changes in the genital microbiota of women in sub-Saharan Africa can influence their reproductive health. Our goal is to better understand the relationship between microbial changes, including the presence of bacterial vaginosis, and a host of factors such as contraceptive use, STIs including HIV and Chlamydia trachomatis, or vulvovaginal candidiasis.
We are further interested in exploring the impact of male circumcision on the bacterial and viral microbiome of the male genital tract to explore mechanisms by which male circumcision reduces the risk of acquiring HIV infection and how various bacterial species and prokaryotic or eukaryotic viruses change the inflammatory state of the male genital tract. We are exploring these and related questions with collaborators in Uganda and South Africa via 16s and metagenomic sequencing.
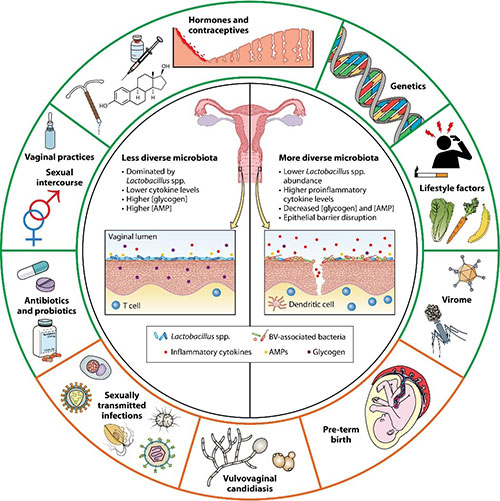
Dabee S, Passmore JS, Heffron R, Jaspan HB. The Complex Link between the Female Genital Microbiota, Genital Infections, and Inflammation. Infect Immun. 2021 Apr 16;89(5):e00487-20. doi: 10.1128/IAI.00487-20. PMID: 33558324; PMCID: PMC8091093.
Partnership Opportunities

Heather B Jaspan, MD, PhD
Heather Jaspan completed her medical degree and PhD in Cell and Molecular Biology at Tulane in the USA, and thereafter did Pediatrics training at the University of Washington/ Seattle Children's Hospital. Upon completion, she returned to Africa, first to Malawi and then back home to South Africa, where she spent 5 years doing clinical HIV prevention research. In 2008, she returned to Seattle Children's to obtain Pediatric Infectious Diseases subspecialty training, returning to basic science immunology research. She spends a large proportion of her time recruiting cohorts in South Africa, and running laboratories in both Cape Town and Seattle, answering immunological questions around HIV prevention in children via breastfeeding/chestfeeding and in adolescents via sexual activity.
-

Colin Feng
Research Lab Supervisor
-

Melanie Gasper, PhD
Research Scientist IV
Melanie’s current work as a research scientist examines innate immune alterations in infants exposed to HIV who remain uninfected, specifically in the context of BCG vaccination.
-

Kira Griswold
Postdoctoral Researcher
-

Dana Kamenz
Research Scientist II
-

Brandon is an Acting Assistant Professor in the Department of Pediatrics. He is currently investigating interactions between the immune system and bacterial and viral microbiota in the male genital tract and the impact of antiretroviral medications on enteric microbiota.
-

