“Future-proofing” against new COVID-19 variants
Online publication date: Aug. 8, 2022
Among the many challenges presented by the COVID-19 pandemic is that by the time a new therapeutic monoclonal antibody — laboratory-made proteins that mimic the body’s immune system to prevent severe illness or death — is approved for clinical use, a new virus variant that can evade it has often already emerged. Thus, the lifespan of these monoclonal antibody therapies has been quite short.
New findings from researchers at Seattle Children’s Research Institute and at the University of Washington School of Medicine suggest a change in the antibody structure might better “future-proof” against new variants, while also enabling a single product to be used to treat patients infected with a broader range of existing variants.
The research, led by Dr. David Rawlings, director of the Center for Immunity and Immunotherapies at Seattle Children’s and a professor in the Department of Pediatrics at the University of Washington School of Medicine, and Dr. Marion Pepper, associate professor and chair of the Department of Immunology at the University of Washington School of Medicine, was published in the Journal of Experimental Medicine.
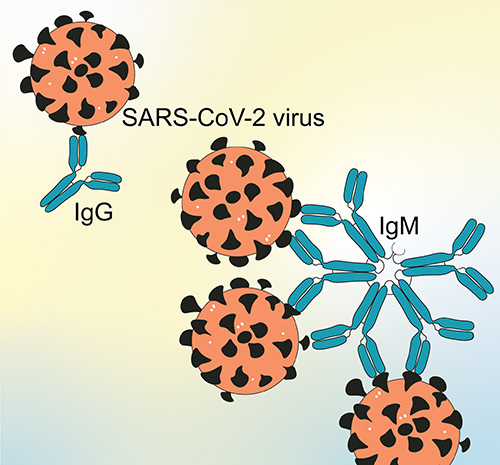 Most of today’s monoclonal antibody therapeutics use a structure called IgG. The most common antibody in blood, IgG is a Y-shaped molecule with two binding sites. However, there is another antibody, IgM, which is shaped like a snowflake (five Y-shapes linked together) with 10 binding sites. By swapping IgG with IgM, the researchers found these COVID-19 antibodies better prevented against infection and against more virus variants, including the omicron variants.
Most of today’s monoclonal antibody therapeutics use a structure called IgG. The most common antibody in blood, IgG is a Y-shaped molecule with two binding sites. However, there is another antibody, IgM, which is shaped like a snowflake (five Y-shapes linked together) with 10 binding sites. By swapping IgG with IgM, the researchers found these COVID-19 antibodies better prevented against infection and against more virus variants, including the omicron variants.
Additionally, the anti-SARS-CoV-2 antibodies used in this research were built from genetic blueprints found in the virus-specific immune memory B cells of individuals who had recovered from COVID-19 infection. The research team found this first-use combination of IgM antibodies derived from memory B cells from previously infected donors worked much better than the IgG version to neutralize the virus, consistent with data they found in an earlier malaria study.
Working with Center for Immunity and Immunotherapies’ Debley Lab, the team was able to demonstrate for the first time the efficacy of an anti-SARS-CoV-2 IgM monoclonal antibody using an advanced cell culture model of the human airway. Using nasal brushings from healthy donors, the lab differentiated patches of airway epithelial cells into complex cultures that closely mimic the physiology of human airway, including production of mucus. When the IgM antibodies were delivered directly to these human airway cultures, a smaller dose was required to block infection than with IgG antibodies.
“This [modeling] expertise is not very common, so having the Debley Lab as a collaborator one floor above us was a unique opportunity to test our most potent IgG and IgM antibodies in a more realistic cell culture model for SARS-CoV-2 infection,” Rawlings said.
The findings have potential to improve vaccine design and lead to the creation of new antibody therapeutics for COVID-19 and other infectious diseases. “The pathogen-specific immune memory B cells that express IgM may be more important for protecting us from future infections than previously anticipated, especially against pathogens that frequently mutate, like SARS-CoV-2,” Rawlings said.
Despite IgM outperforming IgG, IgM has a shorter half-life in the blood than IgG, which may necessitate more frequent dosing. Also, many pharmaceutical companies lack the experience and infrastructure for large-scale production of IgM.
However, the Rawlings Lab and other researchers in the Center for Immunity and Immunotherapies have been working on B cell biology, gene editing and cell therapies for more than a decade.
“We have ongoing projects that are developing engineered B cell therapies that could potentially be given to patients to act as tiny injectable factories for making a therapeutic protein, including IgM,” Rawlings said. Provisional patents have been filed for the novel antibodies, as well as Seattle Children’s Research Institute’s B cell editing technologies.
Seattle Children’s contributing authors include co-lead author Malika Hale, Christopher Thouvenel and Dr. Yu Chen of the Rawlings Lab, as well as Dr. Jason Debley and members of his lab, Lucille Rich and Elizabeth Vanderwall. Researchers from the University of Washington School of Medicine, including co-lead author Dr. Jason Netland, and Harvard Medical School contributed to the work.
The research was supported by a Seattle Children’s Research Integration Hub COVID-19 award, a Burroughs Wellcome Fund pilot grant, funding from the Bill & Melinda Gates Foundation, a Burroughs Wellcome Fund Career Award for Medical Scientists, and grants from the National Institutes of Health.
–Colleen Steelquist
