Promising Seattle Children’s Gene Therapy Offers Hope for Dravet Syndrome
Published
Featured Researchers

Article Summary
New research by Seattle Children’s Research Institute’s Franck Kalume, PhD, and colleagues found a novel genetic approach that could cure Dravet syndrome, a rare and severe form of epilepsy associated with a high rate of sudden, unexpected death. The breakthrough findings are published in the journal Science Translational Medicine.
Dr. Kalume, senior author of the paper and a principal investigator in the research institute’s Norcliffe Foundation Center for Integrative Brain Research, has been researching Dravet syndrome for more than two decades and generated the first preclinical model of the disease. He is considered one of the world’s leading experts in Dravet syndrome research. Over the years, leveraging this disease model, Dr. Kalume and colleagues uncovered deep insights into Dravet syndrome pathophysiology which have served as a critical foundation for the development of a mechanism-based therapy.
Dravet syndrome affects about one in 15,700 children. It usually begins in infancy and causes frequent, prolonged seizures and developmental delays. Current treatment options provide only limited alleviation of symptoms. Patients with Dravet syndrome face a 15% to 20% mortality rate due to sudden, unexpected death, as well as lengthy seizures, accidents and infections.
Mutations in a gene called SCN1A cause most cases of Dravet syndrome. This gene provides instructions to make sodium channel proteins, which play a central role in the generation and propagation of electrical impulses in the brain. In prior work, Dr. Kalume and colleagues showed that Dravet-causing mutations preferentially affect the function of interneurons, with no impact on excitatory neurons. Based on this finding, they predicted that interneurons could serve as a valuable target for a future precision therapy for Dravet syndrome.
This new, proof-of-concept study for gene replacement therapy in Dravet syndrome was co-led by Dr. Kalume and Boaz Levi, PhD, of the Allen Institute for Brain Science. Together with their teams, they developed an approach specifically targeting GABAergic neurons; it showed remarkable protection against key symptoms of Dravet syndrome with no sign of toxicity or side effects. The therapy is delivered using adeno-associated vectors (AAVs), the standard carriers for gene therapy delivery for neurological disorders.
To achieve their results, the researchers had to surmount two critical biotechnological barriers: precise delivery and capacity limits.
Precision Targeting
The first hurdle that the researchers overcame was the lack of an interneuron-targeting approach suitable for AAV-based gene replacement therapy in Dravet syndrome. The team developed a new targeting technology using specialized enhancers to allow precise activation of the therapeutic SCN1A gene only in inhibitory neurons, even though the gene is invariably delivered across all brain cell types.
This is very game-changing. It gives a lot of hope to families of kids with this very severe form of epilepsy. Our hope with this gene therapy is that it will be one shot and done — that we can cure the disease with a single administration.
“We want to treat only cells that are sick or responsible for the disease because giving an extra gene to healthy cells can lead to side effects,” said Dr. Kalume, who is also an associate professor in the Department of Neurological Surgery and an adjunct associate professor in the departments of Global Health and Pharmacology at the University of Washington. “We want to be as precise as possible and only target the disease.”
The ‘IKEA Method’
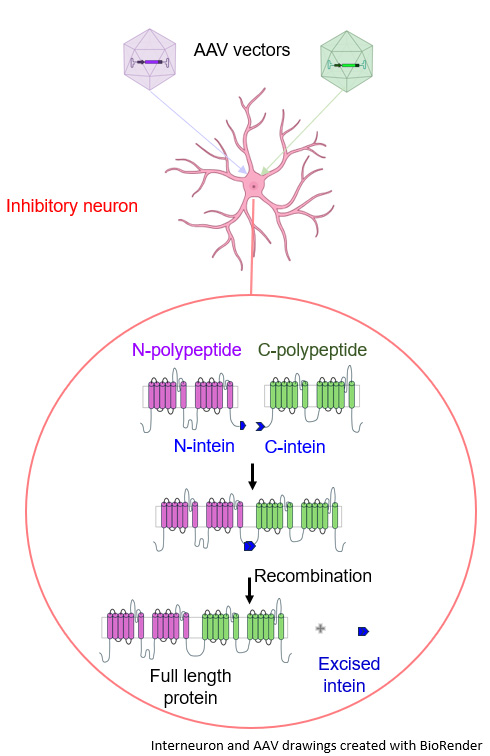 The second hurdle the researchers overcame is that the SCN1A gene size exceeds the cargo capacity of AAVs. So, the team developed a new approach in which the gene is first split in two. Then, the two segments are delivered using two separate AAVs as vehicles and the full-length protein is reconstituted using a fusion technology called intein.
The second hurdle the researchers overcame is that the SCN1A gene size exceeds the cargo capacity of AAVs. So, the team developed a new approach in which the gene is first split in two. Then, the two segments are delivered using two separate AAVs as vehicles and the full-length protein is reconstituted using a fusion technology called intein.
“We call this approach the ‘IKEA method’ because we’re delivering the product in pieces and once we’re at the site, we put it back together,” Dr. Kalume said.
The research team believes this approach could redefine treatment possibilities for Dravet syndrome, as it alleviated key symptoms and enabled long-lasting recovery in the small-animal model.
Hope for a Dravet Syndrome Cure
“This is very game-changing,” Dr. Kalume said. “It gives a lot of hope to families of kids with this very severe form of epilepsy. Our hope with this gene therapy is that it will be one shot and done — that we can cure the disease with a single administration.”
The possibility of a cure deeply motivates Dr. Kalume, who frequently shares research updates with advocacy groups like Partners Against Mortality in Epilepsy, the Dravet Syndrome Foundation and many affected families. Sadly, his interest in preventing epilepsy-related deaths stems in part from personal tragedy.
 Dr. Nino Ramirez
Dr. Nino Ramirez“My own nephew had epilepsy for many years before he was well diagnosed. He passed away at 13 years old. That tragic event has really changed the life of my sister and the whole family, so I understand the fear and worries of parents and how desperately they want to do something about this very devastating form of epilepsy,” he said.
Dr. Kalume hopes to advance the gene therapy from proof-of-concept to a clinical product. He is seeking industry partners and funding to support Investigational New Drug-enabling studies to move the work toward clinical trials.
This study is the result of team science effectively conducted at Seattle Children’s Research Institute. Contributing authors include Jan-Marino (Nino) Ramirez, PhD; Nicole Miranda, PhD (now in the Li Lab); Luiz Oliveira, PhD, Aguan Wei, PhD, and Angela (Michelle) Bard, all of the Ramirez Lab; and Judy (Jiyun) Ryu (formerly of the Kalume Lab).
The research was supported by grants from the National Institute of Mental Health and Seattle Children’s Research Institute and through philanthropy from the Anu and Satya Nadella family.
— Colleen Steelquist
Featured Researchers
Focus Areas
Related Centers & Programs

Publication Q&A: ANKLE2-related Microcephaly: A Variable Microcephaly Syndrome Resembling Zika Infection
Published
Changes in the ANKLE2 gene cause a unique syndrome where children have microcephaly (small brain size) apparent either at birth or shortly after birth, with a broad range of other structural brain abnormalities.
Pinpointing the Origin of Deadly Brain Tumors
Published
In two papers published in Nature, Dr. Kathleen Millen and her team are the first to identify the cells that give rise to the most aggressive forms of medulloblastoma.
Brainstem Region Revealed to Play Role in Regulating Swallow, Breathing
Published
Seattle Children’s researchers revealed novel insights about PiCo, a brainstem region that aids the coordination of swallowing and breathing.

