Current Research
Dysregulated Asthmatic Epithelial Interferon Responses to Rhinovirus Infection
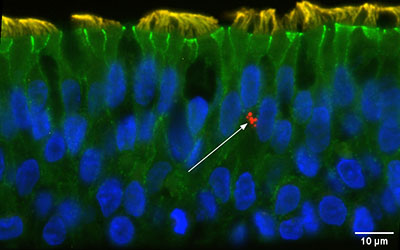
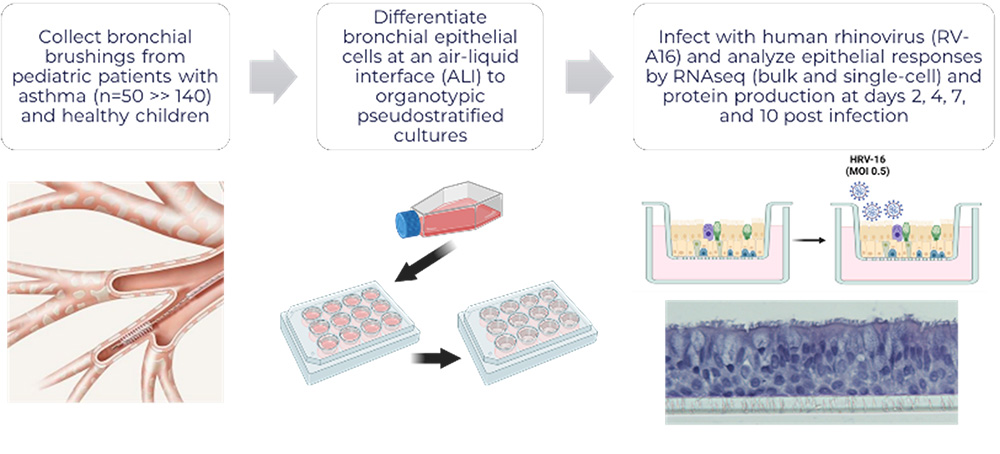
In our cohort of well characterized asthmatic children (funded by NIH/NIAID R01AI163160 Debley- Principal Investigator) from whom we obtain bronchial airway epithelial cells (AECs) and conduct mechanistic ex vivo experiments using organotypic models, we have observed marked heterogeneity in type I and III interferon responses to human rhinovirus (HRV) and RSV infection, with greater HRV replication in AECs from exacerbation-prone asthmatics.
We are testing our central hypothesis that the magnitude and kinetics of AEC IFN responses to HRV influence T2-high and T2-low asthma endotypes, with moderate self-limited IFN responses essential to limit viral replication, reduce exacerbation risk, and dampen T2 inflammation, while exaggerated IFN responses enhance the NLRP3 inflammasome and production of T2-low cytokines (IL-1β, TNF-α), neutrophilic and inflammation, airway remodeling, and lung function decline.
Furthermore, we hypothesize that a common polymorphism in the viral sensor IFIH1/MDA5 (rs1990760), recently associated with asthma, contributes to dysregulated AEC IFN responses to HRV.
Finally, we are utilizing a humanized mouse expressing hICAM1 to allow HRV-A infection, in the context of differential MDA5 function, to test in vivo the role of these pathways in HRV-A infection, T2-high vs. T2-low airway inflammation, exacerbation, and airway remodeling.
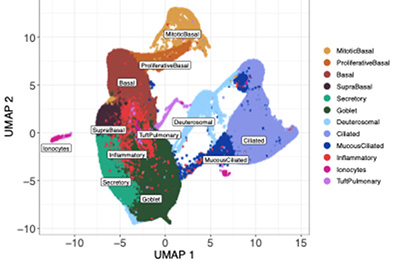 Using bulk and single-cell transcriptomics and proteomics approaches we have identified differentially increased expression of interferon, epithelial remodeling, inflammation, and unfolded protein stress responses by bronchial epithelium from asthmatic children with a severe exacerbation history as compared to asthmatic children without a history of severe exacerbation, and conversely decreased expression of cellular metabolism and transcriptional activity responses by epithelium from asthmatic severe exacerbation as compared to asthmatic donors without severe exacerbations and healthy children.
Using bulk and single-cell transcriptomics and proteomics approaches we have identified differentially increased expression of interferon, epithelial remodeling, inflammation, and unfolded protein stress responses by bronchial epithelium from asthmatic children with a severe exacerbation history as compared to asthmatic children without a history of severe exacerbation, and conversely decreased expression of cellular metabolism and transcriptional activity responses by epithelium from asthmatic severe exacerbation as compared to asthmatic donors without severe exacerbations and healthy children.
Furthermore, we have observed that pre-infection interferon gene expression and secreted protein levels of the epithelial IFN-stimulated chemokine CXCL0 are lower in severe exacerbation donors as compared to asthmatic donors without severe exacerbations and healthy children, and are strongly correlated with greater viral replication post-infection.
Furthermore, prophylactic treatment of epithelial cultures from asthmatic children with IFN-β prior to HRV infection markedly reduces viral load and ameliorates post-infection differential responses between children with and without severe exacerbations.
Epithelial Activation of Airway Inflammation in Patients With Non-T2 Asthma
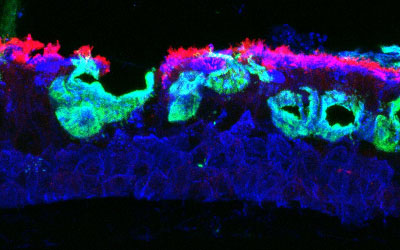 Lower respiratory tract viral infections serve as the dominant trigger for the onset and progression of asthma. Although asthma management has shifted towards targeting airway inflammation at all levels of the disease, primarily with inhaled corticosteroids (ICS) and biologic therapies targeting type-2 (T2) inflammation, there is a substantial burden of disease that is not responsive to these therapies. Specifically, a substantial portion of individuals treated with ICS therapy have persistent T2 inflammation in their lower airways and a large and growing portion of individuals with asthma have inflammation of the airways that is not T2 predominant (non- T2).
Lower respiratory tract viral infections serve as the dominant trigger for the onset and progression of asthma. Although asthma management has shifted towards targeting airway inflammation at all levels of the disease, primarily with inhaled corticosteroids (ICS) and biologic therapies targeting type-2 (T2) inflammation, there is a substantial burden of disease that is not responsive to these therapies. Specifically, a substantial portion of individuals treated with ICS therapy have persistent T2 inflammation in their lower airways and a large and growing portion of individuals with asthma have inflammation of the airways that is not T2 predominant (non- T2).
These related phenomena of persistent T2 (T2-high) and non-T2 inflammation in asthma are emerging as the most common phenotypes in adults, and now make up a substantial portion of children with the disease. The mechanisms responsible for persistent T2 and non-T2 inflammation are incompletely understood, but there is strong evidence that the epithelium and epithelial-derived cytokines play a major role in the immunology of asthma, and we have recently demonstrated that the epithelium is infiltrated with specific innate immune cells that interact with the epithelium to propagate and regulate inflammation.
Our central hypothesis is that the airway epithelium serves as a central coordinator of the immune response to viral respiratory tract infection in asthma. Further, airway epithelial cells (AECs) from T2-high and non-T2 individuals differentially interact with immune cells to support inflammation in a manner that can be identified and targeted.
We have an established program to isolate AECs from children and adults to examine the function of these cells in asthma using a combination of organotypic cell culture models, often in combination with immune cells.
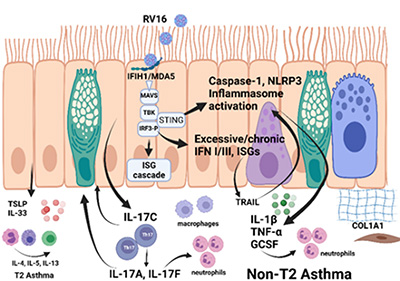
The primary goals of our NIH/NIAID funded Seattle U19 Asthma and Allergic Diseases Cooperative Research Center (AADCRC; U19AI125378 – led by Co-Program Directors Dr. Teal Hallstrand at the University of Washington and Dr. Jason Debley at Seattle Children’s Research Institue/University of Washington) are to identify primary alterations in AECs in asthma, understand how AECs differ between T2-high and non-T2 individuals, and characterize interactions between the epithelium and immune cells that are in close proximity.
In Project 1 (Hallstrand Lab), we examine the underpinnings of persistent T2 inflammation mediated through mast cells and eosinophils acting in conjunction with the epithelium and the components of this inflammation that are resistant to corticosteroids.
In Project 2 (Debley Lab), we examine the basis of interactions between the epithelium and macrophages, Th17 cells, and neutrophils and how epithelial cells from each of the groups support inflammation through these cells.
These projects are supported by Pediatric and Adult Epithelial Cores to isolate AECs from well phenotyped adults and children and further examine connections between phenotype, genomics, genotype, and clinical outcome.
The studies are supported by an Advanced Bioinformatics Core (Altman Lab – Benaroya Research Institute and University of Washington) using state of the art single cell and bulk RNA sequencing.
These studies will facilitate the precise targeting of inflammation based on clinical phenotype and epithelial endotype and a greater understanding of the basis of interactions between the epithelium and immune cells that reside within the epithelium in asthma.
Human Airway Epithelial Cell Culture Core
 The primary goal of Core B Airway Epithelial Cell Culture Core is to support the NIH/NIAID funded Seattle U19 Asthma and Allergic Diseases Cooperative Research Center (AADCRC; U19AI125378) projects by providing organotypic cultures of bronchial airway epithelial cells from carefully phenotyped children and adults.
The primary goal of Core B Airway Epithelial Cell Culture Core is to support the NIH/NIAID funded Seattle U19 Asthma and Allergic Diseases Cooperative Research Center (AADCRC; U19AI125378) projects by providing organotypic cultures of bronchial airway epithelial cells from carefully phenotyped children and adults.
The core is divided into a pediatric sub-core and an adult sub-core. Core personnel identify individuals scheduled for elective surgery, explain the study, and obtain informed consent.
Epithelial cells are collected from enrolled individuals at the time of elective surgery by bronchial brushing via a secure endotracheal tube. Epithelial cells are expanded in submerged culture and either stored or used immediately as needed for differentiated air-liquid interface organotypic cultures to support the co-culture model systems in our Seattle AADCRC projects.
A key function of the Core is to carefully characterize individuals with asthma into T2-high and non-T2 clinical phenotypes, using pre-defined criteria for children and adults. Both sub-cores also collect epithelial cells from healthy controls for comparisons.
Additional longitudinal clinical data is collected from children with asthma, allowing correlation of specific clinical phenotypes and the associated epithelial endotypes with temporal clinical data. These cells will be provided along with assistance from the core for mechanistic studies, using gene disruption techniques such as shRNA gene silencing or CRISPR/Cas9 gene editing.
Impact of Leptin on Bronchial Epithelium in Asthma
Obesity is a known risk-factor for asthma and current treatments for asthma are less effective in patients with obesity. A critical need exists to investigate the molecular underpinning of obesity-related asthma to develop novel therapies.
The cytokine hormone, leptin, has gained attention as a potential mediator in the pathogenesis of complex obese-asthma endotypes. Leptin is primarily produced by adipocytes and is elevated in obesity, and higher leptin serum levels are associated with severe asthma and increased inflammatory markers including IL-6, G-CSF, and NF-κB.
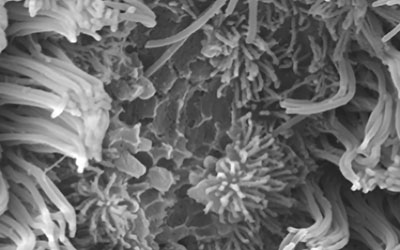 Preliminary data indicate that chronically upregulated leptin signaling promotes increased mucus-secreting cell proportions in the developing epithelium of Xenopus tropicalis and increased mucin release, while downregulated leptin signaling results in inhibited mucus secretion and severely altered mucus-secreting cell morphology. Leptin has been described to increase expression of the rhinovirus adhesion receptor ICAM-1 in epithelial cells, but the impact on viral infection and response has not been investigated.
Preliminary data indicate that chronically upregulated leptin signaling promotes increased mucus-secreting cell proportions in the developing epithelium of Xenopus tropicalis and increased mucin release, while downregulated leptin signaling results in inhibited mucus secretion and severely altered mucus-secreting cell morphology. Leptin has been described to increase expression of the rhinovirus adhesion receptor ICAM-1 in epithelial cells, but the impact on viral infection and response has not been investigated.
Our research objective is to characterize the effects of altered leptin signaling on primary bronchial airway epithelial cells from children with and without asthma and epithelial immune responses to viral infection to test our central hypothesis that increased leptin signaling leads to altered cell composition and function in human airway epithelium.
This collaborative project between the labs of Dr. Jason Debley (SCRI) and Dr. Erica Crespi (WSU) leverages collaborative expertise to integrate a human ex vivo airway epithelial model and transcriptomics at SCRI with microscopy techniques and cellular development characterization at WSU.
CRBT junior faculty member and mentee of Dr. Debley Dr. Weston Powell and WSU graduate student and mentee of Dr. Crespi Kourtnie Whitfield are driving this work forward.
