Molecular Dynamics Lab
Pinpointing How Autism Affects the Brain
Led by Dr. Stephen E.P. Smith, the Molecular Dynamics Lab is working to uncover what the gene variations that contribute to autism have in common, opening the door to a new generation of potential treatments.
Studying a key synapse
Researchers have linked more than 100 gene mutations to autism. But it’s rare to find two children with autism who have the same mutation. This suggests that autism is not a single disease – it’s hundreds of rare, genetic diseases that cause similar symptoms.
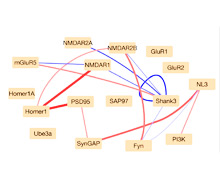
We are looking for similarities in how these diseases affect the brain and change how it functions. Our current research focuses on the brain’s synapses, which make important calculations and help control how the brain and body function.
Each synapse contains a complex web of proteins that work together like tiny molecular machines. Many of the gene mutations linked to autism affect how these protein machines are built. We suspect this might explain why the brains of children with autism function differently than typical children’s brains.
To explore this possibility, we developed an innovative system – called quantitative multiplex immunoprecipitation – that maps out how proteins interact with each other in a synapse. We are using this system to pinpoint how different mutations affect the protein machinery at the glutamate synapse, which is where the brain processes and stores information.
This could help us understand which mutations cause the synapse to malfunction, affecting how children speak, play or interact socially. It could also help us identify when different gene mutations have similar effects on the synapse and how it process information.
Tailored autism treatments
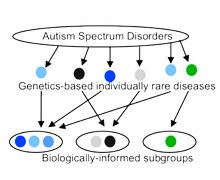 Our long-term goal is to be able to group children with autism into categories based on how their gene mutations affect brain function. This would enable a more precise understanding of how autism varies among children. And it would open the door to new treatments that are tailored to different types of autism.
Our long-term goal is to be able to group children with autism into categories based on how their gene mutations affect brain function. This would enable a more precise understanding of how autism varies among children. And it would open the door to new treatments that are tailored to different types of autism.
Synaptic signaling in autism
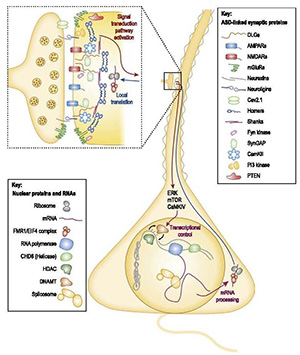
Many autism risk genes converge on signaling at glutamatergic synapses. In the lab we study how these proteins assemble into multi-protein complexes that re-organize during neural activity and plasticity.
We use Quantitative Multiplex co-Immunoprecipitation (QMI) to measure stimulus-dependent changes in protein interaction networks directly in native lysates. This allows us to capture mesoscale signaling logic that expression profiling or phospho-omics may miss.
By comparing multiple ASD models on the same molecular axes, we have identified shared network motifs and biologically meaningful subtypes. We have also shown how homeostatic plasticity rewires the Homer–Shank interactome to adjust excitability.
Together these studies suggest that autism is best understood as a disorder of signal integration at the synapse, with actionable mechanisms at the level of protein complexes rather than single molecules.
mTOR signaling in neurons
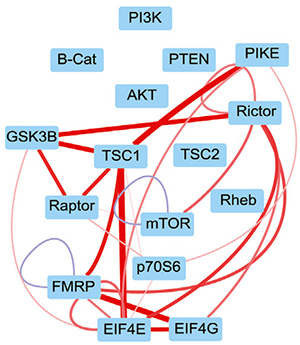 mTOR integrates growth factors, neurotransmitters, and metabolic cues, but neurons use this pathway in ways that differ from dividing cells.
mTOR integrates growth factors, neurotransmitters, and metabolic cues, but neurons use this pathway in ways that differ from dividing cells.
We combine phospho-readouts with QMI to define how neuronal mTOR complexes reorganize in response to different inputs. For example, IGF and glutamatergic stimulation drive distinct rearrangements of mTORC1/2 and translational machinery, including dissociation events that are not predicted from assembly-based models in proliferating cells.
This perspective helps explain how neurons discriminate among convergent inputs and points to new ways of adjusting translation without globally suppressing mTOR.
We are now mapping how disease-linked mutations and homeostatic scaling alter these modules, and testing whether restoring network configuration can normalize function.
CAR T cell signaling in cancer
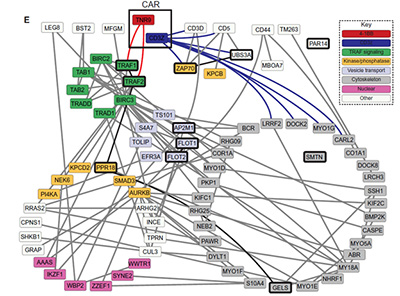 Chimeric antigen receptors were designed to mimic TCR signaling, but in practice their wiring is different.
Chimeric antigen receptors were designed to mimic TCR signaling, but in practice their wiring is different.
We profile the signalosomes that form within minutes of antigen engagement in primary human CAR T cells. Our data have revealed how CARs organize adaptor and kinase modules differently from the native TCR, and how those differences track with variability in cytokine production between manufacturing runs.
These molecular fingerprints can serve both as quality metrics for CAR T production and as a guide for engineering receptor domains that improve potency while reducing toxicities such as cytokine release syndrome.
Towards -omic level study of protein interaction dynamics
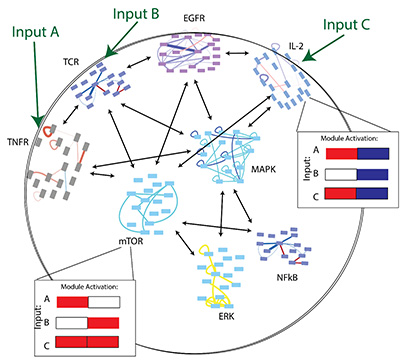 The common thread in our work is that cells compute with modular protein interaction networks.
The common thread in our work is that cells compute with modular protein interaction networks.
We use a stimulus → network reconfiguration → phenotype framework to compare signaling logic: how does a protein network differentiate inputs and compute a response? This approach lets us identify the molecular logic by which cells sense and respond to their environment.
We are currently seeking to scale up our QMI measurement capacity towards a truly -omic scale by generating in-house binding reagents, making parallel measurements of different systems, and incorporating cutting-edge AI language models to define the "language" of the cell via its dynamic protein interaction networks.
Lab News
See all the latest news and publications.
Partnership Opportunities

Stephen E P Smith, PhD
Dr. Stephen E.P. Smith is a principal investigator at the Norcliffe Foundation Center for Integrative Brain Research and an assistant professor at the University of Washington School of Medicine.
-
Emily Brown
Graduate Student
-
Shreya Dev
Undergraduate Researcher
-
Felicia Harsh
Lab Manager
-
Matthew Putnam
Undergraduate Researcher
-
Maisha Sinha
Undergraduate Researcher
