Gallo Lab
The Gallo Laboratory investigates postnatal neural development and how injury or disease affects the growth, regeneration and function of neurons and glial cells. Our multidisciplinary research explores the cellular and molecular mechanisms that shape brain development under both normal and pathological conditions.
We are particularly interested in the signals and pathways that regulate the development of neurons and glia within the white matter, cortex and cerebellum, and in translating this knowledge into strategies for cell repair and regeneration following brain injury.
To achieve these goals, we employ an integrated set of molecular, cellular, anatomical, electrophysiological and behavioral approaches, using animal models that replicate key aspects of human brain injury and disease — including perinatal brain injury (such as hypoxia, maternal immune activation, and oxidative stress), Down syndrome and multiple sclerosis.
A major focus of our work is understanding the response of neural progenitor cells to injury and disease and identifying novel molecular mechanisms that promote neural repair and functional recovery.
More recently, our studies have expanded to combine genetically tractable animal models — which enable precise manipulation of signaling pathways — with investigations in large mammals, whose brain anatomy and cellular organization closely resemble those of humans. We have established preclinical models of perinatal brain injury that mimic the gray and white matter alterations observed in very low birth weight (VLBW) premature infants.
Current projects aim to uncover the cellular and physiological bases of cognitive, behavioral and motor deficits in premature infants, with a special focus on the cerebellum and its roles in brain function. Our ongoing studies integrate findings from animal models and postmortem human brain analyses to advance our understanding of neural development, injury and repair.
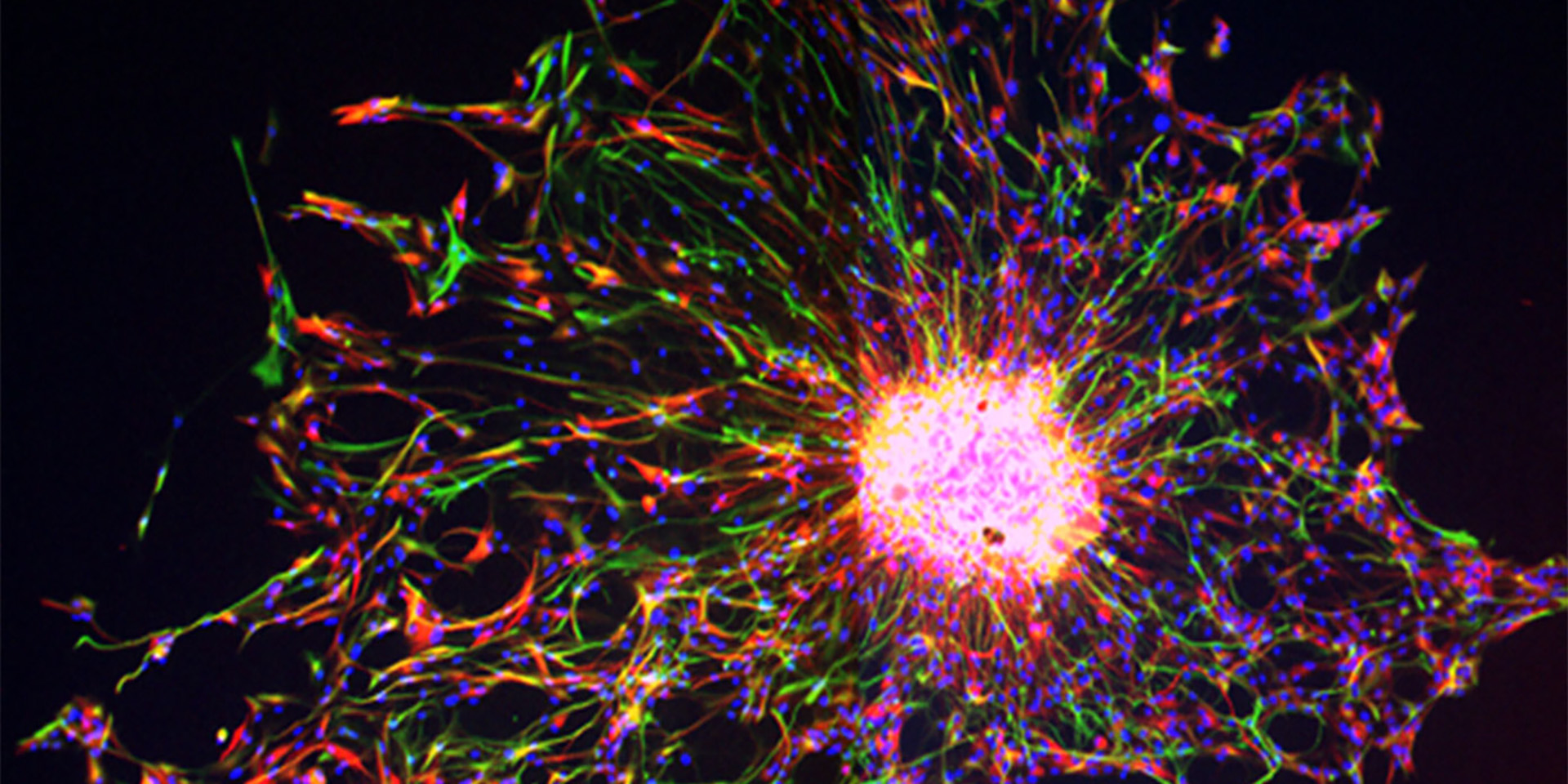
The image above is of the dorsal subventricular zone in an animal model (postnatal day 18) showing expression of Endothelin-1 (green), Glial fibrillary acidic protein (GFAP, red) and cell nuclei (DAPI, blue). GFAP+ astrocytes express the Endothelin-1 protein.

Vittorio Gallo, PhD
Vittorio Gallo, PhD, is senior vice president and chief scientific officer for Seattle Children’s Research Institute and serves as a member of Seattle Children’s Executive Leadership Team.
Dr. Gallo obtained his PhD in Biochemistry and Neurobiology at the University of Rome, Italy, working with Professor Giulio Levi and the Nobel Laureate Professor Rita-Levi Montalcini. He did his postdoctoral work at the MRC Developmental Neurobiology Unit, London (EMBO Fellow) at the Laboratory of Preclinical Pharmacology, NIMH, NIH (Fogarty Fellow), and in the Department of Pharmacology at University College, London (EMBO Fellow).
In 1989, he became visiting scientist and NATO Fellow in the Laboratory of Developmental Neurobiology, NICHD, NIH, and then in 1992 Chief of the Section on Molecular and Cellular Neurobiology at NICHD, where he became a tenured investigator in 1995.
In 2002, he moved to Children’s National Medical Center to become the Director of the Center for Neuroscience Research, where he built a research center focused on neural development and neurodevelopmental disorders. Dr. Gallo served as both interim chief academic officer for Children’s National Hospital and interim director of Children’s National Research Institute. He was also associate dean for Child Health Research, and a professor of pediatrics, pharmacology and physiology at George Washington University School of Medicine and Health Sciences.
Dr. Gallo joined Seattle Children's as senior vice president and chief scientific officer in September 2023.
-

Kritika Bhalla, PhD
Postdoctoral Researcher
Kritika Bhalla, PhD, aims to uncover novel therapeutic targets for the treatment of pediatric brain defective neurocognition and to improve patient outcomes. She is passionate about unraveling the mysteries of the developing brain. Bhalla previously focused on exploring metabolic signaling pathways affected by rare congenital neurodevelopmental disorders. In the Gallo Lab, she investigates the mechanisms underlying defective dysmaturation of oligodendrocyte development and demyelination resulting in the altered motor and cognitive function in Down syndrome. She also explores defects in cerebellar development at cellular, molecular, and anatomical levels during trisomy. With expertise in molecular biology and neuroscience, Bhalla employs cutting-edge techniques to identify molecular pathways implicated in intellectual disability observed in patients with Down syndrome.
-

Dylan Crawford, PhD
Postdoctoral Researcher
Dylan Crawford, PhD, holds a doctorate in psychology from Rutgers University, specializing in behavioral and systems neuroscience. His research expertise lies in investigating gene-environment interactions impacting cognitive abilities alongside a focus on anxiety-like behaviors in a small-animal model. His postdoctoral work at Rowan University explored cognitive flexibility in Alzheimer’s disease and repeated TBI. His current research has pivoted to the bioinformatic and behavioral assessment of sepsis treatment in small-animal models, as well as developing innovative cognitive paradigms and learning multi-omics analytic techniques. Crawford is affiliated with professional societies like IBNS, Psi Chi and the National Postdoctoral Association. Driven by a passion for science communication, he wants to scientific knowledge accessible through teaching, outreach and diverse communication channels.
-

Julian Naizaque
Research Scientist III
Julian Naizaque has long been captivated by the brain’s complexity and how neurological diseases can alter not only its function, but also who we are as human beings. He joined Dr. Orlando Torres-Fernandez’s group at the Instituto Nacional de Salud in Bogota, Colombia, to study neurological vulnerabilities induced by neurotropic viruses. During his undergraduate biology and graduate neuroscience studies, he researched the effect of rabies virus on the expression of calbindin and parvalbumin in the cerebellum. He also contributed to studying the Zika virus’ effects on the development of the cerebral cortex and cerebellum. He’s currently focused on the pathological mechanisms involved in neonatal brain injury in the cerebellum, studying how white matter injury could alter Purkinje cell morphology and its effects on oligodendrocyte lineage progression.
-

Alejandro Parga-Becerra, MD, PhD
Scientific Group Leader
Alejandro Parga-Becerra, MD, PhD, is an experienced neurophysiologist with a background in trauma medicine and advanced proficiency in clinical and experimental methodologies, including vector interventions and opto/chemogenetics. He is a leader in the orchestration of complex research initiatives aimed at the evaluation and modulating of brain function. Skilled in integrating neuroanatomical, electrophysiological, and multiomics approaches to decipher neuronal circuits, he is proficient in guiding multidisciplinary teams in academia and industry, with a focus on platform/technology development. His overarching objective is to elucidate the mechanisms of neuronal ensembles crucial to cognitive processes and to leverage this knowledge to drive technological innovations for enhancing cognition.
-

Emma Parkins, PhD
Postdoctoral Researcher
Emma Parkins, PhD, studies white matter development and injury in a small-animal model of preterm birth. She is building on previous work to identify the intrinsic and extrinsic mechanisms that underlie protracted white matter immaturity observed in neonatal hypoxia, including the role of synaptic dysfunction in delayed oligodendrocyte progenitor cell proliferation and maturation. She received her doctorate from the University of Cincinnati Neuroscience Graduate Program, where she worked in the lab of Dr. Christina Gross at Cincinnati Children’s Hospital to study the role of microRNAs in sculpting excitatory postsynapses in the hippocampus. Before that, she studied neuroscience and gender, sexuality and feminist studies at Oberlin College, where she researched multisensory integration in autism spectrum disorder.
-

Olivia Santiago
Research Scientist I
After earning her bachelor’s degree in biology at Lewis & Clark College in 2020, Olivia Santiago was a research assistant in a diabetes and obesity lab at the University of Washington, where she helped manage the small-animal models and collaborate on various projects using transgenic small-animal models to investigate the molecular mechanisms of the brain that control feeding, weight gain and blood glucose homeostasis. She is excited to develop her research skills in the Gallo Lab and expand her neuroscience knowledge into perinatal brain injury and repair.
Panagiotis Kratimenos, MD, PhD
Center for Neuroscience Research
Children’s National Research Institute
Division of Neonatology
Children’s National Hospital
Ioannis Koutroulis, MD, PhD, MBA
Center for Genetic Medicine Research
Children’s National Research Institute
Division of Emergency Medicine
Children’s National Hospital
Nobuyuki Ishibashi, MD
Center for Neuroscience Research
Children’s National Research Institute
Children’s National Hospital
Tarik Haydar, PhD
Center for Neuroscience Research
Children’s National Research Institute
Children’s National Hospital
Jeff Dupree, PhD
Department of Anatomy and Neurobiology
Virginia Commonwealth University
Developmental Myelination and Myelin Regeneration
