Rajagopal Lab
Welcome to the Rajagopal Lab
In the Rajagopal Lab, we utilize genetic, molecular, biochemical and proteomic approaches to study infectious diseases caused by bacteria. The human pathogens that we study are group B Streptococcus and Staphylococcus aureus. Although both GBS and S. aureus are commensal organisms, these bacteria can also become disease-causing pathogens.
Our research interest is to understand signaling events and gene regulation that are critical for disease pathogenesis of GBS and S. aureus.
GBS Hemolytic Toxin Research
Research on group B Streptococcus are focused on understanding how the pathogen migrates through different host niches and interactions that are critical for disease progression or bacterial clearance. Research includes identifying pathogenic factors important for disease and host cells and mechanisms critical for immune defense. We hope that a greater understanding of the molecular mechanisms involved during bacterial pathogenesis will enable us to identify novel compounds that can be used to treat these bacterial infections.
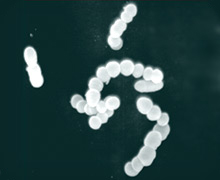
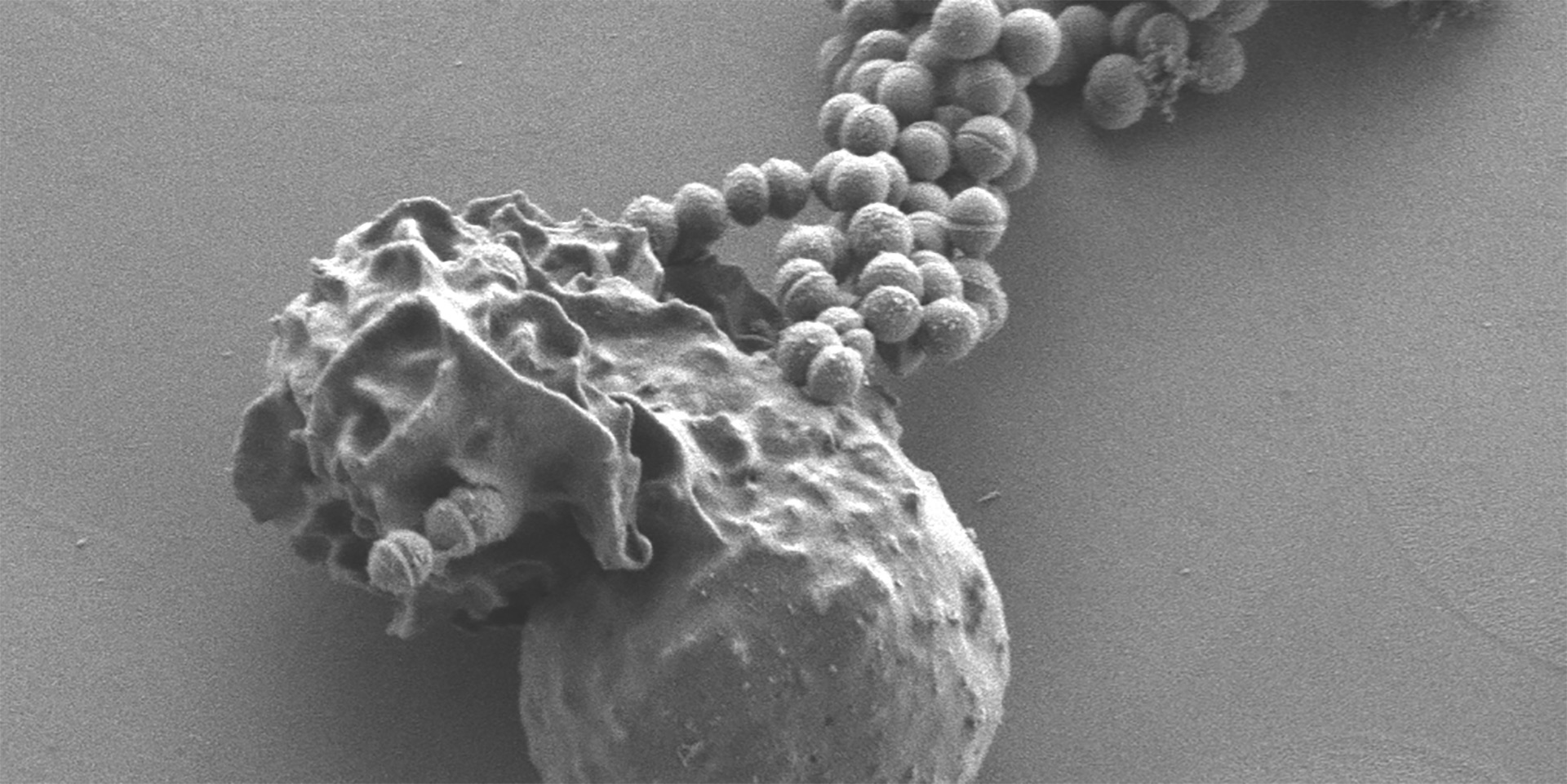
Group B Streptococcus (GBS) are commonly found in the recto-vaginal tract of healthy women but can cause severe invasive disease in human newborns and adults – including elderly, immunocompromised and diabetic individuals. GBS disease commonly manifests as pneumonia, sepsis and meningitis. Our studies on GBS are focused on understanding how these bacteria adapt to the environmental niches encountered during their life cycle. Studies from our research have shown that GBS encodes signaling factors such as a serine/threonine kinase; these proteins were previously thought only to exist in higher forms such as eukaryotic organisms. Our research showed that the kinase regulates the expression of the GBS toxin known as hemolysin/cytolysin and also enables the bacteria to adapt to nutrient starvation. We have demonstrated that the kinase affects toxin expression based upon its interaction with a DNA-binding response regulator known as CovR. The interaction between the kinase and CovR represents novel findings in bacterial environmental adaptation. More recently, we have shown that GBS mutants defective for CovR signaling show increased blood-brain barrier penetration and thus provide insight into how the organism can transition from a commensal organism to a virulent, meningeal pathogen. Current research is focused on identifying the environmental cues/signals that are sensed by these bacteria for regulation of toxins and understanding how the pathogen migrates through different host cells during infections.
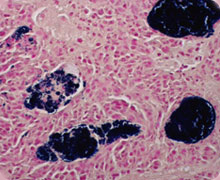 Like GBS, S. aureus are also Gram-positive bacteria that can cause severe invasive disease in humans. Infections due to S. aureus include deep-seated infections such as bacteremia, sepsis, pneumonia, ostemolyelitis and toxic shock syndrome. In the U.S., infections caused by methicillin-resistant strains of S. aureus (MRSA) have even surpassed numbers linked to HIV/AIDS. We have recently identified a number of novel genes/signaling factors that regulate antibiotic resistance, toxin expression and S. aureus virulence. Current studies are focused on elucidating how these signaling factors affect antibiotic resistance and S. aureus infections such as sepsis and pneumonia. We are also investigating how mutations in host signaling pathways affect disease susceptibility to S. aureus. This is particularly relevant as patients with genetic disorders such as Jobs syndrome and chronic granulomatous disease (CGD) are prone to recurrent and life-threatening infections due to S. aureus. The ultimate goal is to use the information gathered from our research to identify novel compounds that can be used to treat these bacterial infections.
Like GBS, S. aureus are also Gram-positive bacteria that can cause severe invasive disease in humans. Infections due to S. aureus include deep-seated infections such as bacteremia, sepsis, pneumonia, ostemolyelitis and toxic shock syndrome. In the U.S., infections caused by methicillin-resistant strains of S. aureus (MRSA) have even surpassed numbers linked to HIV/AIDS. We have recently identified a number of novel genes/signaling factors that regulate antibiotic resistance, toxin expression and S. aureus virulence. Current studies are focused on elucidating how these signaling factors affect antibiotic resistance and S. aureus infections such as sepsis and pneumonia. We are also investigating how mutations in host signaling pathways affect disease susceptibility to S. aureus. This is particularly relevant as patients with genetic disorders such as Jobs syndrome and chronic granulomatous disease (CGD) are prone to recurrent and life-threatening infections due to S. aureus. The ultimate goal is to use the information gathered from our research to identify novel compounds that can be used to treat these bacterial infections.
In July 2014, Rajagopal received a five-year, $2.4 million grant from the National Institutes of Health to study how the pigment/lipid toxin activates an immune response and causes host cell lysis. This will be valuable for the development of therapeutic strategies that prevent Group B streptococcal infections in humans.
Previous research
Published in the Journal of Experimental Medicine in 2013, Rajagopal determined the hemolytic toxin found in GBS was not a protein, as previously believed, but a different cell structure known as a lipid. The finding may prevent the development of a vaccine for GBS, because the molecular structure of lipids prevents the toxin from being inactivated by antibodies – the traditional way that vaccines neutralize toxins made of protein molecules. The group has also shown that the GBS hemolytic toxin destroys neutrophils and evades neutrophil extracellular traps in the placenta leading to fetal injury and preterm labor (Sci Immunol 2016;1(4):aah4576) and have used analogs to mitigate hemolytic GBS mediated disease.
Learn more:
Partnership Opportunities
Lakshmi Rajagopal, PhD
Dr. Rajagopal is a professor in the Department of Pediatrics, Division of Infectious Disease at the University of Washington. She has adjunct faculty appointments in the Departments of Microbiology and Global Health at the University of Washington. She is also a full faculty member of the Molecular and Cellular Biology PhD program of the University of Washington.
Rajagopal is a member of the Center for Global Infectious Disease Research at Seattle Children's Research Institute, where her laboratory is physically located. She is an internationally recognized expert on the role of novel signaling pathways in Gram-positive pathogens.
Funding for her research is in part supported by the NIAID-NIH.
-

Michelle Coleman, PhD
Research Scientist
-

Austyn Orvis
Research Scientist
-

Ravin Seepersaud, PhD
Research Scientist
-

Shams Sidhamed
Research Technician
-

Rishita Singh
Student Helper
-

Joy Twentyman
Postdoctoral Researcher
