Kaushansky Lab
Our Best Hope in Fighting Global Disease Is Open Collaboration
Infectious diseases remain a substantial cause of mortality around the world. This is particularly true in resource-poor areas, where access to treatment is sparse and people fight a multitude of infections simultaneously. In the case of malaria, hundreds of millions are infected every year, and the disease takes the lives of half a million people annually. Yet the malaria parasite and other pathogens that burden the world cannot survive independently. To cause sickness and travel through the population, they must appropriate resources from the people they infect. Our work aims to identify what pathogens need from their host and to use this knowledge to prevent and eliminate infectious diseases.
Dr. Alexis Kaushansky is a research biologist, but her experience in engineering school and social justice issues before grad school has made her a scientist who looks at social and economic constraints as a path to creativity. She has implemented innovative techniques and made some startling discoveries in the quest for a malaria cure — discoveries that might help us fight other infectious diseases as well.
Malaria Host–Parasite Interactions in the Liver
When malaria parasites are transmitted from mosquito to human, they are first deposited into the skin, then quickly travel to the liver. In the liver, each parasite replicates tens of thousands of times within the confines of a single hepatocyte. During this stage of infection, the parasite causes no clinical symptoms, yet elimination of the parasite in the liver prevents disease and transmission and can even elicit sterile immunity from subsequent infection. Failure to stop the parasite during the liver stage leads to the release of parasites into the bloodstream. When in the bloodstream, parasites infect red blood cells and cause the clinical symptoms of malaria, including severe fever, anemia, organ failure, coma and even death.
Our work focuses on the basic question of how the malaria parasite is able to modify its liver environment in order to counteract host defenses and ensure its own survival. The malaria parasite is able to alter its environment quite significantly, yet only some of these parasite-induced changes are critical for the parasite to survive. We use a variety of approaches and technologies to identify the changes that are initiated by the malaria parasite, and hone in on the environment the parasite requires for survival.
Malaria Host–Parasite Interactions in Cerebral Malaria
In rare but deadly cases of malaria infection, the parasite causes breakdown of the blood-brain barrier, leading to brain swelling. This is known as cerebral malaria and is most commonly diagnosed in children under the age of 5. It is also extremely deadly: even with current front-line therapies, 15 to 20% of kids who get cerebral malaria die of the disease. Even for those who recover, long-term impacts are common. We aim to better understand what happens during this disease, and how it is shaped by the complex reality in the field, where some kids are exposed to malaria by the bite of an infected mosquito every day.
Host-Based Drug Discovery
Our research is driven by a deep curiosity about the fundamental properties by which pathogens control their hosts. We are inspired to focus our efforts on malaria and other infectious diseases because of their massive worldwide impact. By identifying specific needs that the malaria parasite has in the liver, we can target these host factors with drugs and eliminate the malaria parasite.
Many of the host factors the malaria parasite uses in the liver are already the subject of well-established drug development efforts. Targeting host factors that impact the parasite is far less likely to lead to the development of drug-resistant parasites. This approach is in sharp contrast to existing antimalarial drugs, which target the malaria parasite directly and thus require de novo development. The host-targeted approach is particularly well-suited to malaria, which afflicts people who, on average, live on two dollars a day, and to which drug resistance is widespread.
Pathogen-Based Drug Discovery
One-third of the global population faces the constant threat of infection by malaria-causing Plasmodium vivax parasites. P. vivax infection is unique in that it results in dormant forms that remain in the liver for weeks to months before reactivation and recurrence of blood-stage infection. The existing treatment landscape for P. vivax dormant liver stages is severely limited, as current drugs are unsuitable for a significant portion of the population in endemic areas, including pregnant women and individuals with glucose-6-phosphate dehydrogenase deficiency. The escalating challenge of drug resistance to traditional antimalarials further compounds this limited treatment landscape. Consequently, there is a critical need for new therapeutic options to effectively mitigate the global health and economic impacts of malaria.
Our work focuses on developing novel compounds to drive the inhibition of N-myristoyltransferase (NMT), a crucial enzyme in eukaryotic organisms. Although Plasmodium NMT is essential for lifecycle progression, and inhibition has been shown to eliminate parasites, previous attempts at developing P. vivax-specific NMT (PvNMT) inhibitors have been unsuccessful due to low selectivity and crossover inhibition of human NMTs. Our work aims to overcome the limited treatment landscape for dormant forms of P. vivax by developing effective PvNMT inhibitors that have high parasite selectivity and are effective at eliminating parasites at multiple stages of their life cycle.
Insectaries
Our work and the work of colleagues critically depend on malaria parasite infection in mosquitoes and the production of sporozoites for lab experiments. We maintain state-of-the-art insectaries that breed and house Anopheles mosquitoes. Mosquitoes are infected with rodent malaria parasites and with Plasmodium falciparum, which are maintained under safe containment conditions. Malaria parasite mosquito stages are then isolated and used for experimentation. Infected mosquitoes are also used for malaria challenge studies of human volunteers in our Malaria Clinical Trials Center. Learn more about the CGIDR insectaries.
Beyond Malaria
While many of the projects in our laboratory have started with an interest in malaria, because we often develop tools and technologies to enable new experimental lines of research, we often have the opportunity to work collaboratively to apply these lines of new technologies to other areas. As one example, we have developed a series of innovative computational tools to elucidate the host factors, particularly protein kinases, that control pathogen infection and development in their hosts. These tools are broadly applicable and have been applied to traumatic brain injury, sepsis, dengue infection and many other areas.
Sporozoite Extraction Automation
Malaria remains a substantial worldwide health problem. The Plasmodium parasite, the causative agent of malaria, develops into its human-infectious form in the salivary glands of female Anopheles mosquitoes. To perform research on Plasmodium transmission and initial development within the liver of the human host, researchers must dissect the salivary glands of hundreds of mosquitoes each week. There are malaria vaccine candidates that also rely on the frequent and speedy extraction of live parasites from mosquito salivary glands. The process of hand dissection is laborious for research and limits the ability to increase vaccine production to the scale needed for worldwide administration.
The Problem
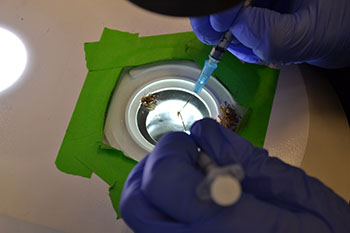 Mosquito dissections are a complex, tedious, and time-consuming task. Each mosquito has six salivary glands located where its head meets its thorax. Salivary glands of infected mosquitoes contain mature Plasmodium parasites. Researchers dissect out the salivary glands from each mosquito and put them into a tube where they will be ground to release the parasites. During dissection, careful attention is paid to avoid the mosquito midgut, located in the abdomen, which contains sporozoites that are both less infectious and less immunogenic than salivary gland sporozoites.
Mosquito dissections are a complex, tedious, and time-consuming task. Each mosquito has six salivary glands located where its head meets its thorax. Salivary glands of infected mosquitoes contain mature Plasmodium parasites. Researchers dissect out the salivary glands from each mosquito and put them into a tube where they will be ground to release the parasites. During dissection, careful attention is paid to avoid the mosquito midgut, located in the abdomen, which contains sporozoites that are both less infectious and less immunogenic than salivary gland sporozoites.
The salivary glands can be difficult to identify because of their size and difficult to separate from the rest of the mosquito because of their stickiness. The parasites can only survive outside of their host for a limited time, so the dissections need to happen quickly. Many projects are limited by the number of mosquitoes researchers can dissect. Significant training is involved for researchers to feel confident in their accuracy and speed. It can take months for users to feel comfortable and lots of practice to increase their dissection speed. Dissections are such a daunting and variable task; some labs are discouraged from researching liver-stage malaria.
Goals for Improvement:
- Ease workflow
- Improve efficiency
- Decrease training time
- Ease the burden of entry into malaria research
- Increase the pace of vaccine production
Ideation
Standard dissection involves many precise and case-specific movements, so our first goal was to reduce the process to simple and repeatable motions that would be easy to automate in the future. To organize our ideas, we broke down the process into steps (collecting mosquitoes from their cage, transporting them to the device, dissecting, and cleaning and resetting the device) and listed ideas for how each could be achieved. The first important step of dissection is proper alignment of the mosquito. Typically, the mosquitoes are collected into a 50mL conical of ethanol and moved into a small mesh filter where they bunch up. We thought that we could take advantage of the mosquitoes’ normal resting position and capture them in a way where they are consistently set in a predetermined position.
Our first design was a cartridge-based system. We used vacuum to pull mosquitoes through a tube into a removable cartridge. The goal was to have the mosquito aligned such that its head would be peaking out, ready for decapitation.
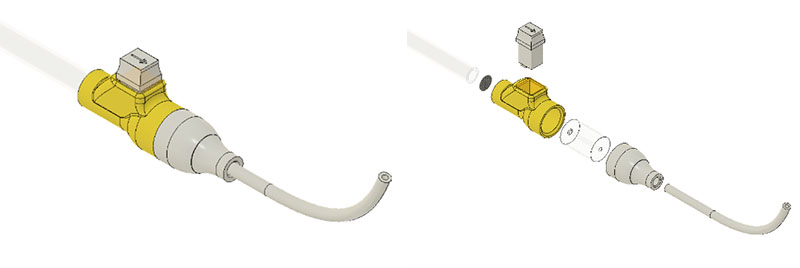

Different cartridge designs: straight slot, tapered slot, straight slot with cover, tapered slot with cover. A properly aligned mosquito in a cartridge (straight slot with cover).
The mosquito alignment was working consistently so we moved toward increasing the amount of mosquitoes we could align at one time. We switched the cartridge design from a rectangular one to a cylindrical one, and then created the rotating cartridge system. This system had the capacity to hold eight mosquitoes.

Next, we played around with different ways to remove the salivary glands, trying to replicate the two main methods done by hand: pulling the head off slowing with the glands attached and then separating them off, and cutting the head off, pressing down on the thorax, and teasing out the glands from the rest of the guts.
We played around with sonication as a way to dissect mosquitoes. We experimented with sonicating different parts of the mosquito (whole mosquito, head & thorax, head & glands, just glands), and found that we could isolate the most parasites from whole mosquitoes and head & thoraxes. We knew we wanted to eliminate the mosquito abdomen because it has fewer infectious parasites, so we decided to move forward with devices to separate head & thoraxes.
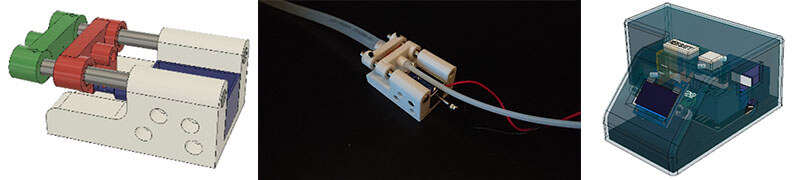
Semi-automated thorax slicer that uses a solenoid to open chamber where mosquito gets lodged.
Machined a thorax slicer to reduce binding.
Components
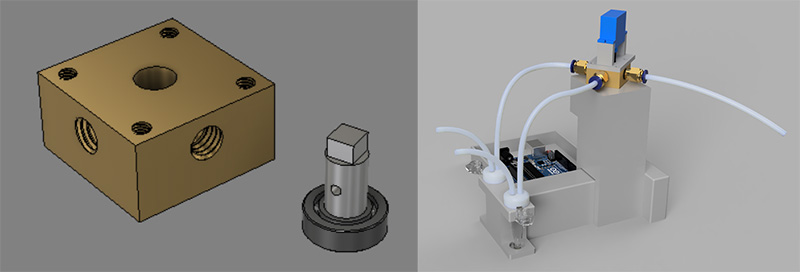
The final design of the squito splitter uses a rotary shear mechanism to separate the head & thorax of the mosquito from its abdomen.
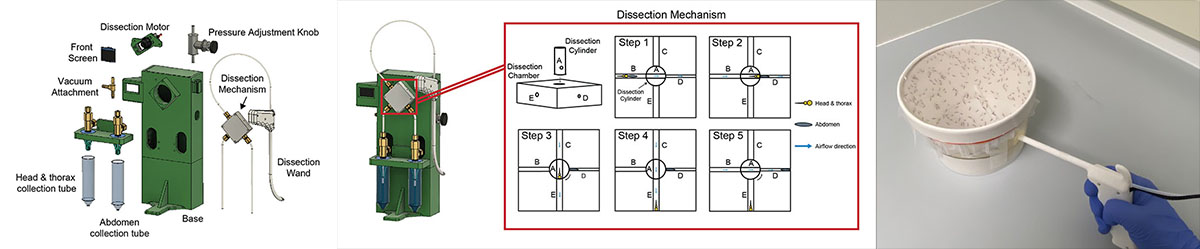
The Problem
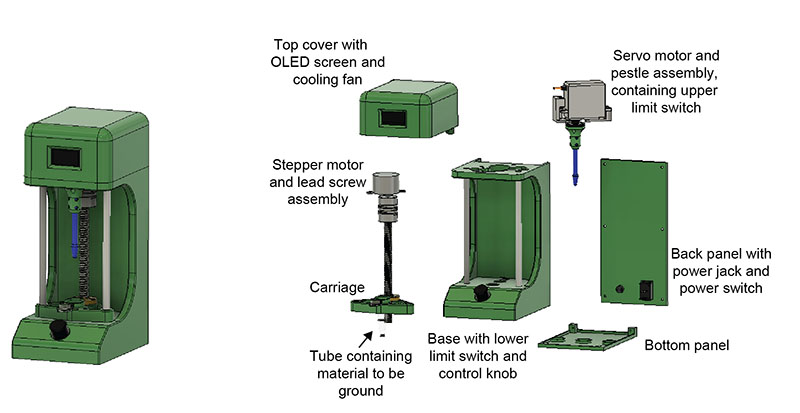
Plasmodium sporozoites are traditionally isolated from mosquito salivary glands by manually grinding mosquito material in an Eppendorf tube using a pestle. This process is tedious and highly variable between trials and between researchers. Our goal was to standardize grinding approaches and reduce researcher workload.
Components
The final design replicates a mortar and pestle. The user places the tube in the carriage and uses the control knob to choose the number of grinds. The stepper motor raises the carriage up to the pestle and the servo motor spins the pestle, after which the tube is lowered back down. The screen displays the number of grinds in real time.
Insectary Automation
Live mosquitoes are required to comprehensively study vector borne diseases. Standard rearing methods are tedious and time-consuming, and often generate inconsistent results. We have developed a series of systems to automate the rearing and handling of Anopheles stephensi mosquitoes with the goal of increasing the ease, efficiency, and accessibility of mosquito production.
The Problem
A crucial part of mosquito rearing is the daily draining of larval pans to harvest pupae. Each pan must be lifted and emptied into a 5-gallon bucket where a strainer collects the mosquito pupae and larvae.

The 40-pound bucket of dirty water then gets hoisted and dumped into the sink. We can empty up to 200 pans in one day which equals about 70 gallons of water. The frequent emptying of this bucket (around 14 times/day) is unpleasant and exhausting.
Components
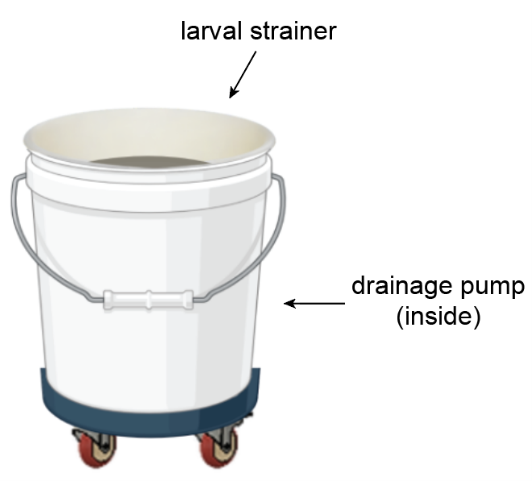
We improved this task by placing a pump inside the bucket and putting the whole system on wheels. When the water inside the bucket reaches a certain level, the pump automatically activates and empties the water directly into the sink—no lifting necessary. The wheels enable the bucket to be easily rolled around the insectary. Both of these components allow insectary workers to empty pans without interruption, greatly reducing the time and physical energy required.
The Problem
As mentioned in the section above, a huge part of the insectary workload is emptying up to 200 larval pans a day in order to harvest pupae. Lifting pans of water is already an awkward and tedious task, but doing it in an extremely hot and humid environment makes it even more challenging and hazardous.

After each pan is emptied, larvae are poured back in, and the pans need to be refilled with water—another laborious task. Our goal was to create a system that could reduce manual labor and human variation leading to a more efficient, consistent, and pleasant rearing experience.
Scale Model
Before building a huge new system, we wanted to create a mock-up model using the rearing pans we already had.

We built this mini version and reared mosquitoes in it for several weeks, testing out different angles for the pans and different methods for rinsing out every last larva. Once we were happy with the design, we began researching materials and components for the full-scale pupation station.
Build Process


Components
The final design is a semi-autonomous system we call the pupation station that has the capacity to empty and refill with the click of a button. It is an array of large aluminum pans (6ft x 4ft) with electronically actuated valves. Each pan is angled slightly to help the water drain out quickly. The direction of the tilt and the placement of the valve alternates with each pan. The whole system is on wheels in case it needs to be moved.
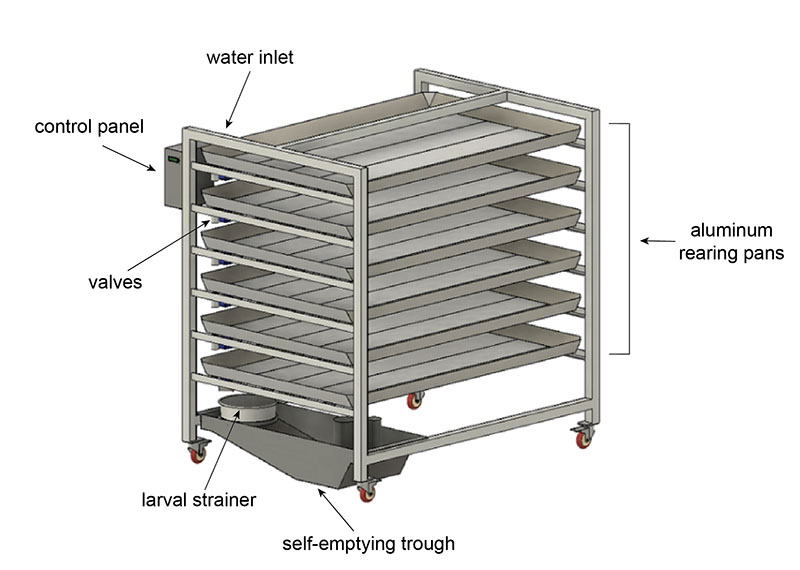
When the user wants to drain the system, they use the control panel to select the “empty” function. All valves will open, and the pan contents will start to drain out. There is a hose hooked up so that the user can easily spray water to guide all the larvae out. At the bottom of the system is a strainer that catches the larvae and pupae. The water flows into the trough which functions exactly like the self-emptying bucket—a pump automatically activates and drains the water into the sink. Once the pans have drained, the user can select the “fill” function to refill all the pans with the perfect amount of fresh water.

This system simplifies the rearing process and reduces the workload compared to manual methods. The pupation station reduces the time spent emptying pans per week by two-thirds and completely eliminates heavy lifting. It also increases the consistency of the process, which maximizes mosquito yields.
The Problem
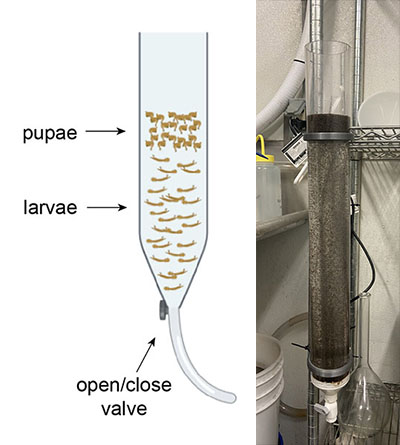
 The standard method for separating pupae from larvae is called “cold watering” and includes pouring the mosquitoes into a volumetric flask and filling the bottom half with room temperature water and the top half with cold water. The pupae will rise to the top and can be poured off and put into cages. The larvae will remain at the bottom, and once the pupae are off, they can be poured back into the pans to continue developing.
The standard method for separating pupae from larvae is called “cold watering” and includes pouring the mosquitoes into a volumetric flask and filling the bottom half with room temperature water and the top half with cold water. The pupae will rise to the top and can be poured off and put into cages. The larvae will remain at the bottom, and once the pupae are off, they can be poured back into the pans to continue developing.
The volumetric flask has some limitations. The mosquitoes need oxygen to breathe, and the very small opening on the flask means that the mosquitoes cannot survive in there for long. The separation can happen very quickly with a small number of mosquitoes, but as our production has increased, we have noticed die-off among the larvae when they were kept in the flask for too long. Our goal was to design a device with an easy way to get larvae out fast and with a bigger diameter so that more larvae and pupae could get oxygen.
Components
This manually controlled pupae separation funnel simplifies and improves the cold-watering process. When cold water is added, the pupae start rising to the top and the larvae sink to the bottom. This funnel has a valve at the base which allows the user to separate off the bottom layer of larvae and place them back into rearing pans within 30 seconds, reducing their time without oxygen. Instead of waiting for all the pupae to be poured off before the larvae can return to the rearing pans, this device makes it so that the bottom larvae can be released quickly without disrupting the cold-watering process. The diameter of the funnel is larger than that of the volumetric flask, allowing for greater access to oxygen.
The Problem
After the pupae are harvested, they are poured into petri dishes with water and then placed into cardboard cages. Once the pupae have all emerged into adults, it is important to remove the water from the petri dishes. This process is an important step for maintaining the cage environment because leftover mosquito debris can decompose and lead to contamination if it is not removed.

Cage with a new pupal dish (left). Cage where nearly all the mosquitoes have emerged—the dish is ready to be emptied (middle). Cage with pupal dish that has been properly emptied and closed (right).

The standard method for emptying pupal dishes is to use a serological pipette pump, which pulls liquid up as one spins a gear with their thumb. We would carry all the cages that need to be emptied from the incubator to the sink and empty them one by one. The serological pipette would need to be inserted into the cage 3 or 4 times in order to pull out all the water. This process is tedious, slow, and messy.
Components
We designed a simple vacuum system that allows for the rapid emptying of pupal dishes. This system consists of a suction tube, a waste flask, and a vacuum pump. The whole system can sit right next to the incubator, so there is no need to move the cages anywhere.
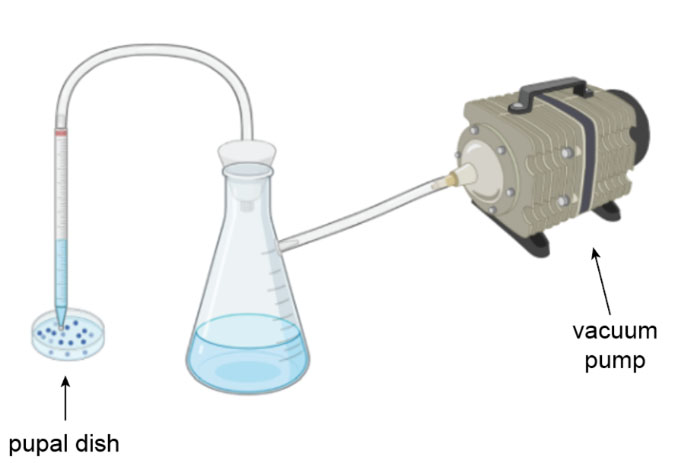 The user turns on the vacuum, opens the incubator, and inserts the suction tube into a cage. Without turning off the vacuum, the user moves the tube from one cage to another. This system allows for quick and continuous dish draining at a rate of 5 seconds per cage compared to the manual rate of 45 seconds per cage.
The user turns on the vacuum, opens the incubator, and inserts the suction tube into a cage. Without turning off the vacuum, the user moves the tube from one cage to another. This system allows for quick and continuous dish draining at a rate of 5 seconds per cage compared to the manual rate of 45 seconds per cage.

The Problem
To transfer mosquitoes from one cage to another, researchers often use mouth aspirators. Not only is it unsanitary to put one’s mouth on laboratory equipment, but it is also uncomfortable and easy to make mistakes. We wanted to create a system that would transfer mosquitoes safely and efficiently, without using our mouths.
Components
The system consists of a suction lid for the destination cage, a vacuum pump, and a transfer tube. The user places the suction lid on the destination cage and puts one end of the transfer tube into the destination cage. After turning on the vacuum, the user puts the other end of the transfer tube into the cage with mosquitoes—the decrease in pressure in the destination cage pulls the mosquitoes through the transfer tube into the destination cage.
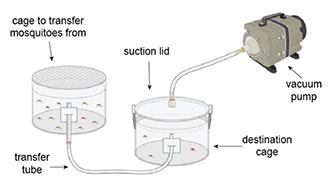
This device enables quicker transfer of mosquitoes and is easier and safer for the user. The direct transfer tube is a significant improvement over the mouth aspirator when considering mosquito survival because mosquitoes are not compacted against each other during transfer.
The Problem
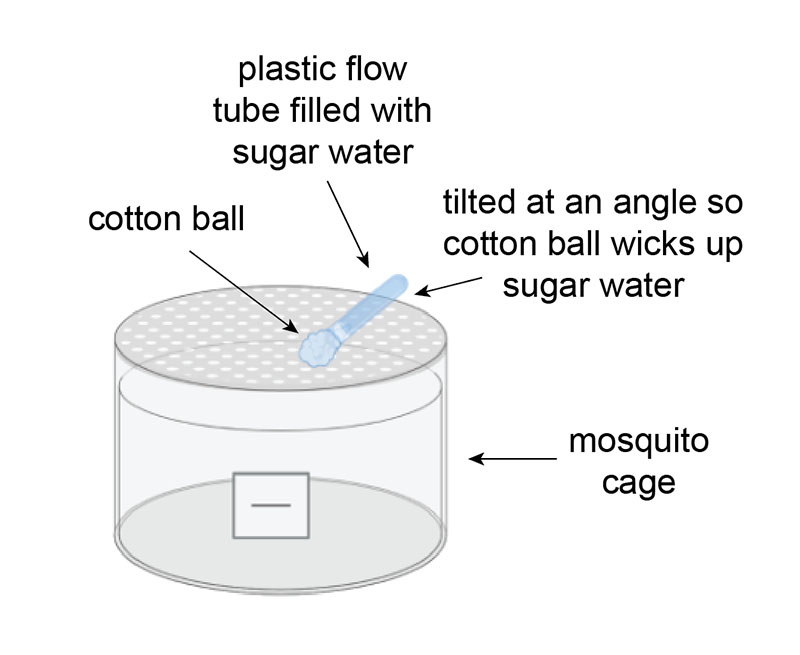
The standard method of feeding mosquitoes is soaking cotton balls in sugar water and placing them on top of the mosquito cage. The cotton balls typically dry out within 24 hours and need to be replaced on a daily basis. We were looking for a solution that would increase the amount of time the cotton balls would stay moist, specifically to avoid coming in over the weekend just to feed.
Components
This new setup consists of a plastic flow tube filled with sugar water and plugged with a soaked cotton ball. The sugar tube is placed at an angle against the rim of the cage so that the cotton ball lays flush with the netting and the back end of the tube sticks up. As the cotton ball dries out, it wicks up the sugar water in the tube, remoistening the cotton ball. This setup triples the amount of time the cotton ball stays wet.
Partnership Opportunities

Alexis Kaushansky, PhD
Alexis Kaushansky, PhD, is a professor at, and interim director of, the Center for Global Infectious Disease Research. She received her BS in chemistry from Harvey Mudd College in 2004, and her PhD from Harvard University in 2010. During her PhD, she trained with Dr. Gavin MacBeath, where she was fortunate to work as part of an interdisciplinary team that included chemists, computer scientists and biologists to develop protein array technology, and apply this approach to understanding the molecular changes that occur in cancer. She joined Seattle Children's Research Institute as a postdoctoral fellow in 2010 to use similar technologies to uncover changes that occur in the liver in response to malaria infection. In 2015, she started a lab at the center which is focused on discovering how pathogens, such as malaria, interact with their human host and using this knowledge to eliminate infection. Outside of work, she enjoys running, cooking, traveling and debating current events.
-

Rossana de la Noval
Grants and Contracts Supervisor
-

Luana Dos Santos Ortolan, PhD
Research Scientist III
-

Cassandra Fieldson
Research Associate I
-

Elizabeth Glennon, PhD
Research Scientist IV, Supervisor
-

Nhi Ho
Graduate Student
-

Tony Isebe, PhD
Invent@SC Postdoctoral Research Scholar
-

Thalia Solyanik
Research Scientist I
-

Tinotenda Tongogara
Research Scientist I
-

Elzani van Zyl, PhD
Invent@SC Postdoctoral Research Scholar
-

Lola Velush
Student Helper
-

Ganesh Ram Visweswaran, PhD
Research Scientist IV
-

Ling Wei, PhD
Research Scientist III
