Nontuberculous Mycobacteria
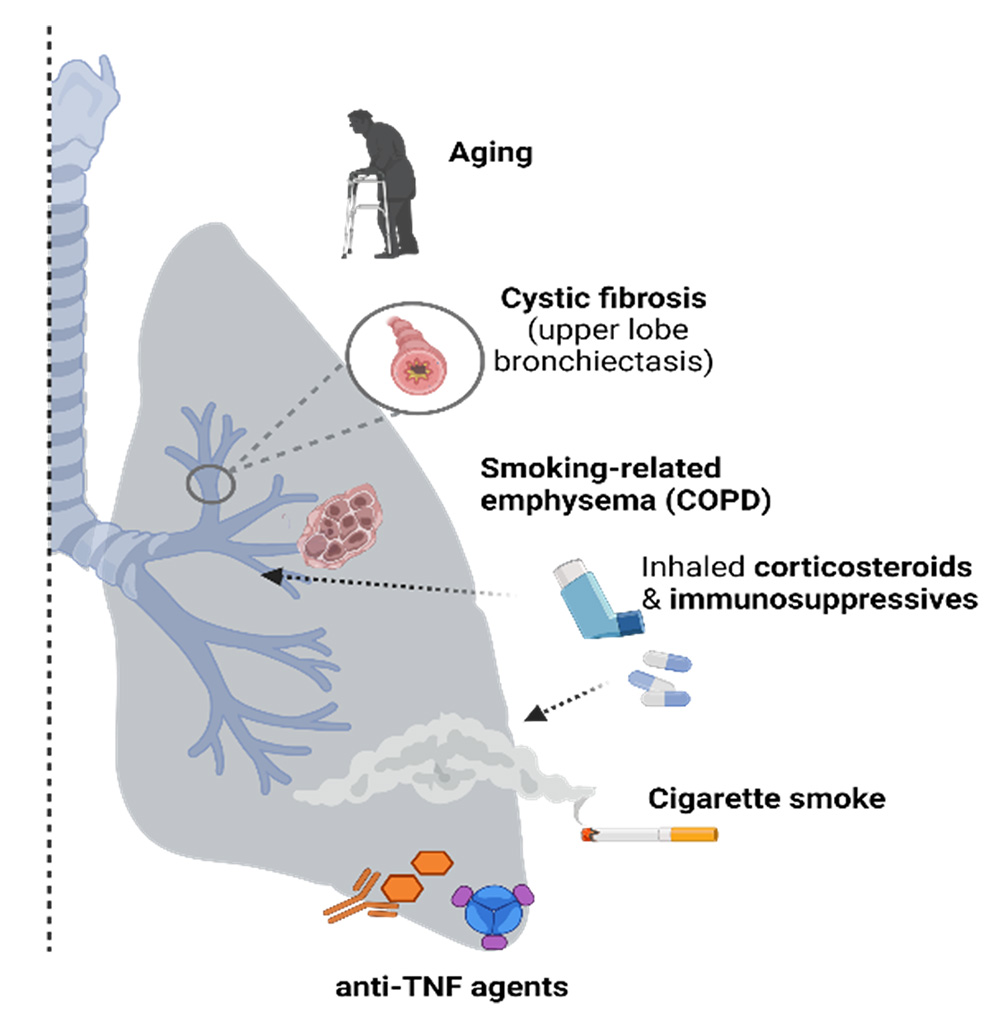
Targeted risk factors for NTM-lung disease.
U.S. prevalence of Nontuberculous mycobacterial-lung disease (NTM-LD) is 3-6 per 100,000 and appears to be increasing. Patients at risk for developing NTM-LD include those with preexisting lung conditions or immune perturbations such as (i) chronic obstructive pulmonary disease (COPD), (ii) immune senescence (aged individuals), and (iii) patients requiring anti-TNF treatments against inflammatory diseases such as rheumatoid arthritis.
M. abscessus (M.abs) is an opportunistic mycobacteria that often colonizes the lung airways in patients with cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), or bronchiectasis, whereas humans (and mice) with normal lung airways are typically resistant to lung infection and NTM. Our work on this proposal is an extension of our systematic and methodical approach towards controlling diseases caused by Mycobacterium sp. Over the last 25 years, we have successfully developed viable anti-mycobacterial candidates such as M72/AS01E and ID93+GLA-SE which are both in clinical stage testing.
This project is designed to develop an efficacious and durable prophylactic anti-NTM candidate targeted towards individuals at risk for developing NTM-LD. The candidates that will be tested in this proposal include 16 antigens with known importance in Mycobacterium avium (M.av) and Mycobacterium abscessus (M.abs) infection that have critical roles in bacterial entry, growth, virulence, enhanced survival and stress conditions within the host. Antigens will be down-selected based on their recognition (and cytokine production) by NTM-infected human peripheral blood mononuclear cells (PBMCs). In addition, the NTM candidate antigens will be screened using a search for homology to >1,700 clinical NTM isolates. We plan to characterize the responses of immune-enhancing agents by evaluating innate immune cytokine production with varying doses of these agents in combination with the NTM antigens, as well as testing their ability to kill the mycobacteria using activated macrophage and dendritic cell-killing assays. Prior to testing the vaccine candidates in our challenge models, we plan to include a mycobacterial growth inhibition assay (MGIA) that will be adapted to NTM.
By including innovative approaches and models, this will project will accelerate new NTM treatment regimens. This R01 project will develop a prophylactic anti-mycobacterial treatment candidate against clinical NTM isolates, including both M.abs and Mycobacterium avium (M.av) and will offer an alternative strategy to drug treatment in at-risk individuals.
