Immunotherapeutic approach for the prevention of secondary infection with drug-resistant TB
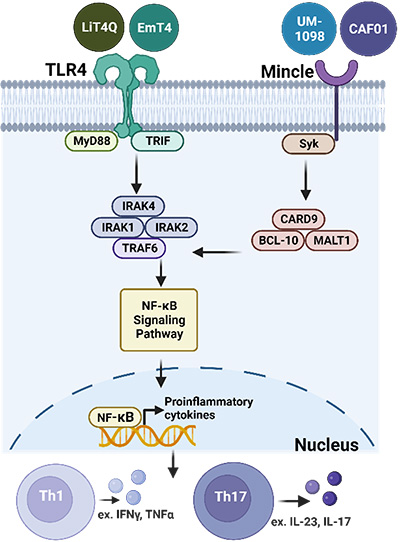 Toll-like receptor 4 or Mincle signaling and induced adaptive immune responses.
Toll-like receptor 4 or Mincle signaling and induced adaptive immune responses.
Many models suggest that specifically targeting prevention of active disease and limiting reinfection with Mycobacterium tuberculosis (M.tb) would have the largest global population-level impact against the deadliest infectious disease. Despite this, many pipeline and preclinical models continue to focus on prevention of primary infection or reduced bacterial burden as stage-gating criteria for candidate vaccine advancement.
Here we propose a more focused and translational screen of therapeutic vaccine candidates designed to be used in conjunction with drug treatment for post-primary tuberculosis (TB). These studies focus on leveraging the models and knowledge obtained through our more than 20 years of antigen discovery and adjuvant development.
This proposal will investigate a host-directed therapeutic approach using antigens that have been successfully vetted in human clinical trials or with preclinical efficacy against M.tb, many of which were developed by our team into novel antigen fusion proteins used in M72 (Rv1196 and Rv1025) and ID93 (Rv2608, Rv3619, Rv3620, and Rv1813) vaccine candidates. Furthermore, our candidate design includes adjuvants without the historical fate of those significantly paused due to competing first-world supplies or those trapped behind intellectual property rights. TLR4 and Mincle adjuvants will be supplied for this proposal by leaders in the field with a commitment to global access (PAI Life Sciences, HDT Bio, CRODA and the University of Montana).
We hypothesize that by including antigens that cover several stages of M.tb infection and that have been previously vetted clinically, preclinically and in the context of M.tb exposure, this will lead to a highly successful therapeutic vaccine candidate.
Our strategy:
- Characterize lead vaccine candidates and components.
- Screen candidate vaccines for therapeutic efficacy against M.tb
- Evaluate the top vaccine candidates in a secondary model for TB.
- Test the lead candidate for prevention of reinfection and/or disease using a barcoded M.tb system.
- Use the collaborative cross mouse model to decipher the genetic contribution to therapeutic protection from a lead vaccine candidate.
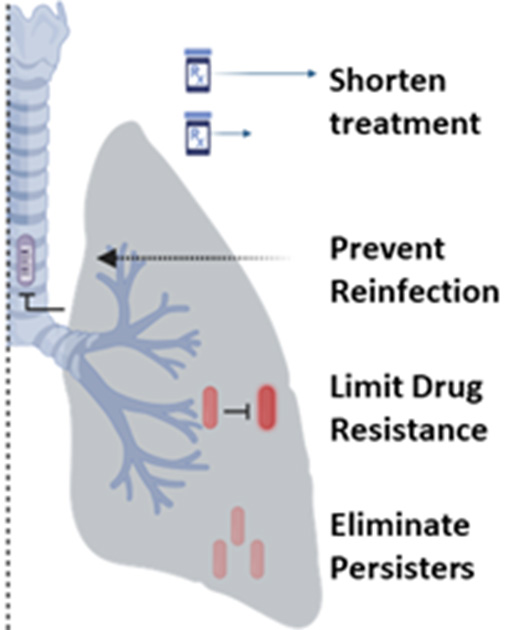 Possible treatment outcomes using a combined therapeutic vaccine and drug treatment regimen.
Possible treatment outcomes using a combined therapeutic vaccine and drug treatment regimen.
