Current Research at the Coler Lab
Immunotherapeutic approach for the prevention of secondary infection with TB
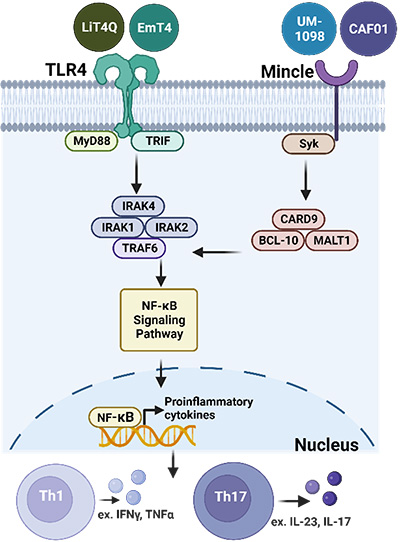 Toll-like receptor 4 or Mincle signaling and induced adaptive immune responses.
Toll-like receptor 4 or Mincle signaling and induced adaptive immune responses.
Many models suggest that specifically targeting prevention of active disease and limiting reinfection with Mycobacterium tuberculosis (M.tb) would have the largest global population-level impact against the deadliest infectious disease. Despite this, many pipeline and preclinical models continue to focus on prevention of primary infection or reduced bacterial burden as stage-gating criteria for candidate vaccine advancement.
Here we propose a more focused and translational screen of therapeutic vaccine candidates designed to be used in conjunction with drug treatment for post-primary tuberculosis (TB). These studies focus on leveraging the models and knowledge obtained through our more than 20 years of antigen discovery and adjuvant development.
This proposal will investigate a host-directed therapeutic approach using antigens that have been successfully vetted in human clinical trials or with preclinical efficacy against M.tb, many of which were developed by our team into novel antigen fusion proteins used in M72 (Rv1196 and Rv1025) and ID93 (Rv2608, Rv3619, Rv3620, and Rv1813) vaccine candidates. Furthermore, our candidate design includes adjuvants without the historical fate of those significantly paused due to competing first-world supplies or those trapped behind intellectual property rights. TLR4 and Mincle adjuvants will be supplied for this proposal by leaders in the field with a commitment to global access (PAI Life Sciences, HDT Bio, CRODA and the University of Montana).
We hypothesize that by including antigens that cover several stages of M.tb infection and that have been previously vetted clinically, preclinically and in the context of M.tb exposure, this will lead to a highly successful therapeutic vaccine candidate.
Our strategy:
- Characterize lead vaccine candidates and components.
- Screen candidate vaccines for therapeutic efficacy against M.tb
- Evaluate the top vaccine candidates in a secondary model for TB.
- Test the lead candidate for prevention of reinfection and/or disease using a barcoded M.tb system.
- Use the collaborative cross mouse model to decipher the genetic contribution to therapeutic protection from a lead vaccine candidate.
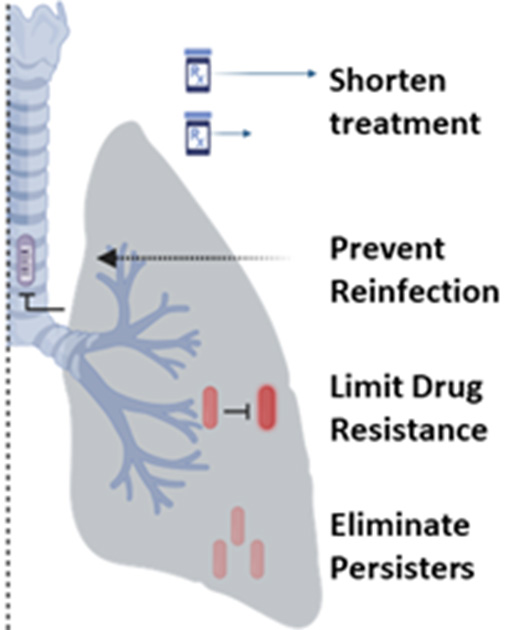 Possible treatment outcomes using a combined therapeutic vaccine and drug treatment regimen.
Possible treatment outcomes using a combined therapeutic vaccine and drug treatment regimen.
Phoenix IMPAc-TB
The Immune Mechanisms of Protection Against Mycobacterium tuberculosis Centers (IMPAc-TB) program is an initiative established by NIAID in 2019 to elucidate the immune responses needed to protect against infection with Mycobacterium tuberculosis (M.tb). The program will lead to a better understanding of tuberculosis (TB) immunology, which is critical to guide the design and development of new and improved TB vaccines, and it aligns with the goals of the NIAID Strategic Plan for Tuberculosis Research (see a PDF of the full strategic plan).
Under this initiative, two of the three IMPAc-TB contracts were awarded to principal investigators at Seattle Children’s Research Institute:
- Rhea Coler, MSc, PhD, Seattle Children’s Research Institute: Coler Lab
- Kevin Urdahl, MD, PhD, Seattle Children’s Research Institute: Urdahl Lab
- Sarah Fortune, M.D., Henry Boom, M.D., JoAnne Flynn, Ph.D.
Phoenix IMPAc-TB contract under Dr. Rhea Coler
The goal of the Phoenix IMPAc-TB project is to comprehensively identify the complex immune responses required to prevent M.tb infection or active TB disease by comparing and interrogating the mycobacteria- and vaccine-induced protective immunity in several animal species and in man.
The overarching paradigm of this project is that identification and validation of common protective correlates of immunity against M.tb (a NIAID Category C pathogen) in escalating preclinical animal models of TB, nontuberculous mycobacteria (NTM) exposure, and human challenge experimental medicine clinical studies, will be crucial in the rational design and development of candidate vaccines that generate robust levels of durable, protective immunity against TB. We have assembled a team of researchers from eight academic or translational institutions who are leaders in various fields, including preclinical animal models of TB, human challenge models, clinical and systems immunology, genomics, microbiology, statistical and bioinformatics methodology, nanoparticle formulation, cGMP manufacturing through fill and finish, regulatory, quality control, clinical trials, and development of linked platform technologies and immunology assessments.
Coler Lab Phoenix IMPAc-TB team at Seattle Children’s Research Institute
 Hazem Abdelaal
Hazem Abdelaal
Fellow PhD
 Sabiriin Abdi
Sabiriin Abdi
Research Technician I
 Sasha (Larsen) Akins
Sasha (Larsen) Akins
Research Scientist IV
 Susan Baldwin
Susan Baldwin
Research Scientist, Senior
 Bryan Berube
Bryan Berube
Research Scientist IV
 Rhea Coler, MSc, PhD
Rhea Coler, MSc, PhD
 Matthew Harband
Matthew Harband
Research Technician I
 Shumei Jiang
Shumei Jiang
Research Associate II
 Suhavi Kaur
Suhavi Kaur
Research Technician II
 Dana Miller
Dana Miller
Research Technician II
 Tiffany Pecor-Korman
Tiffany Pecor-Korman
Research Associate III
 Thomas Smytheman
Thomas Smytheman
Research Technician II
 Alison Wald
Alison Wald
Scientific Project Manager I
 Lindsey Warner
Lindsey Warner
Fellow PhD
 Zhiyi Zhu
Zhiyi Zhu
Research Scientist II

Phoenix IMPAc-TB partner principal investigators
- Andrew Fiore-Gartland
- Sally Sharpe
- Cecilia Lindestam Arlehamn
- Edward Chan
- Marcela Henao-Tamayo
- Brenden Podell
- Helen McShane
- Ryan McNamara
Bacteriophage
Bacteriophages (phages) have been minimally used in emergency clinical applications for skin and soft tissue infections and disseminated bacterial infections, but not to-date as an aerosol strategy against pulmonary mycobacteria. One limitation in the field preventing these advancements is the availability of a reproducible preclinical model of aerosol phage delivery that can be leveraged for efficacy testing. Importantly, our preliminary proof-of-concept results1 (read the paper) suggest that prophylactic aerosol delivery of phage can significantly reduce bacterial burden after challenge with M.tb. This work is done extensively in partnership with the Hatfull Lab at the University of Pittsburg. Support for the mycobacteriophage program has come from NIAID grant funding (now complete) and by the Gates Foundation (active).
Our bacteriophage interests include:
- Using high throughput screening methods to evaluate the lytic capacity of phage candidates against pathogenic bacteria, including M.tb, NTM and bacteria known to affect persons living with Cystic Fibrosis. Our systems allows testing in a range of strains and conditions, including hypoxia, titrations of MOI, in combination with antibacterial drugs and over a range of timepoints. We believe evaluating therapeutic phage candidates across a physiologic conditions will help facilitate translation to the clinic.
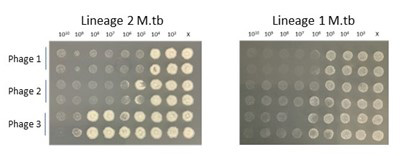
- We are dedicated to developing reproducible preclinical aerosol models of delivery and answer longstanding biological questions about phage treatment in vivo. This includes basic host immunological outcomes of repeated phage treatment, route of delivery and pharmacodynamics/kinetics. Our data suggest that repeated aerosol delivery limits the development of anti-phage humoral immunity and deposits higher titers in the airway when compared to intravenous treatment (read the paper).
These preclinical models are currently being leveraged to evaluate a cocktail of phages designed to treat M.tb in partnership with collaborators under the Phages4TB consortium, supported by the Gates Foundation.
-
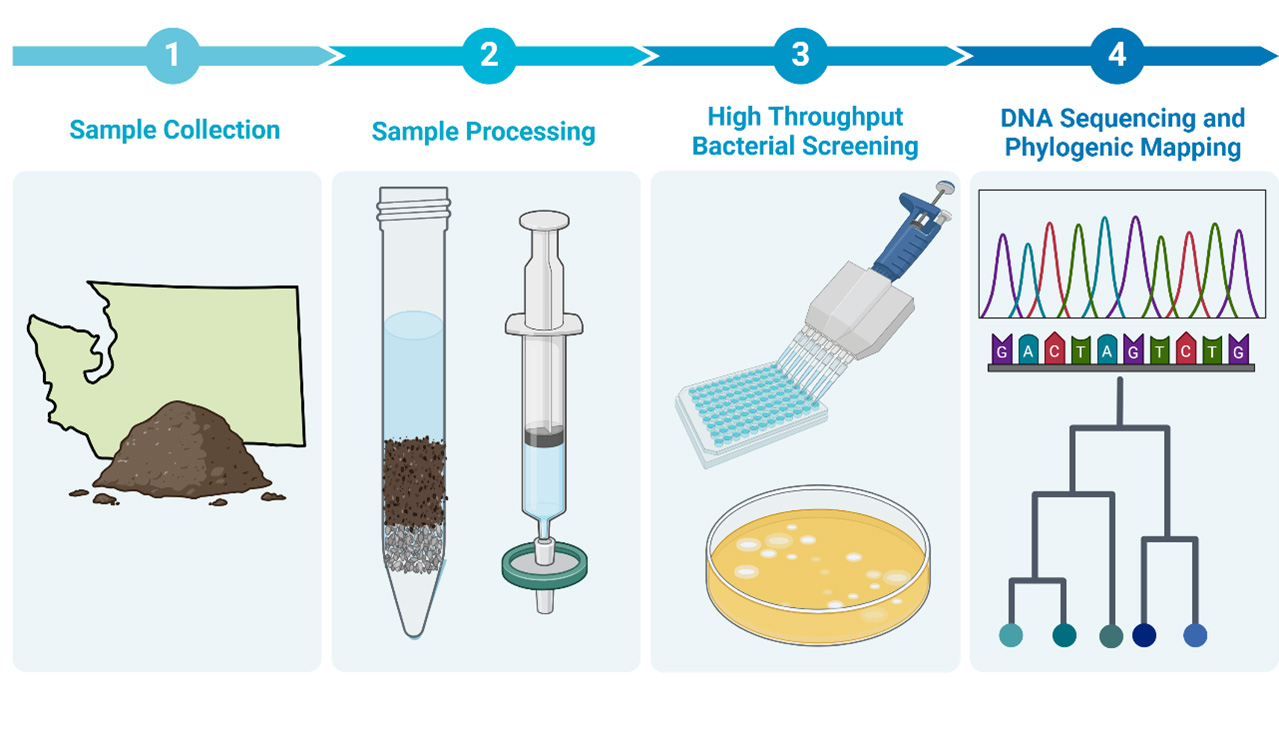
The Pacific Northwest region has contributed a minor fraction of mycobacteriophages to the Actinobacteriophage Database at PhagesDB.org (a Hatfull Lab managed resource). Our group performs regular phage hunts using environmental samples collected throughout the year to try and fill this void.

Samples containing prospective phage are screened against nonpathogenic and subsequently pathogenic bacterial isolates. Hits are sequenced and deposited with Phagesdb.org. We subscribe to the model that science should be equitable and accessible so we regularly extend phage hunt invitations to university student groups, scientific and non-scientific staff at CGIDR as well as visiting scientists and interns.
Questions or interest in the bacteriophage program should be directed to Sasha Larsen Akins, Ph.D. ([email protected]).
Nontuberculous Mycobacteria
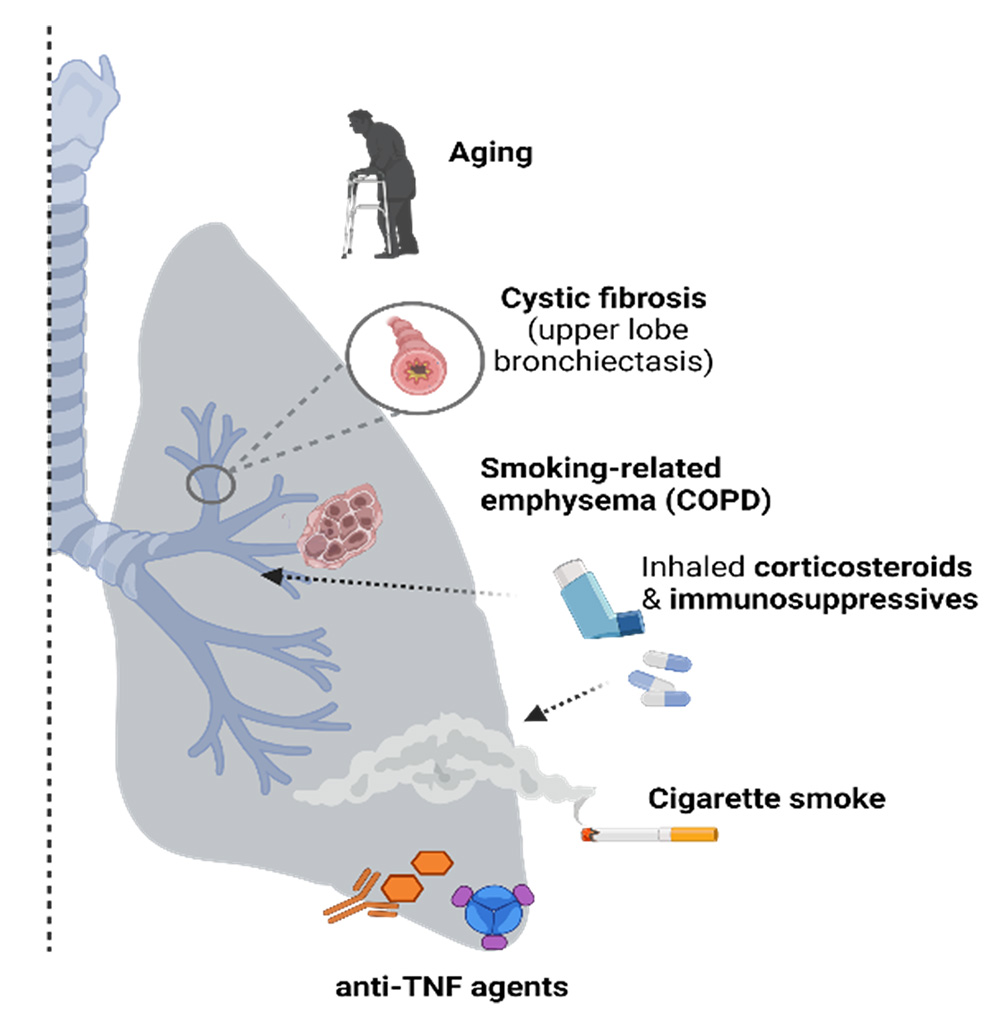
Targeted risk factors for NTM-lung disease.
U.S. prevalence of Nontuberculous mycobacterial-lung disease (NTM-LD) is 3-6 per 100,000 and appears to be increasing. Patients at risk for developing NTM-LD include those with preexisting lung conditions or immune perturbations such as (i) chronic obstructive pulmonary disease (COPD), (ii) immune senescence (aged individuals), and (iii) patients requiring anti-TNF treatments against inflammatory diseases such as rheumatoid arthritis.
M. abscessus (M.abs) is an opportunistic mycobacteria that often colonizes the lung airways in patients with cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), or bronchiectasis, whereas humans (and mice) with normal lung airways are typically resistant to lung infection and NTM. Our work on this proposal is an extension of our systematic and methodical approach towards controlling diseases caused by Mycobacterium sp. Over the last 25 years, we have successfully developed viable anti-mycobacterial candidates such as M72/AS01E and ID93+GLA-SE which are both in clinical stage testing.
This project is designed to develop an efficacious and durable prophylactic anti-NTM candidate targeted towards individuals at risk for developing NTM-LD. The candidates that will be tested in this proposal include 16 antigens with known importance in Mycobacterium avium (M.av) and Mycobacterium abscessus (M.abs) infection that have critical roles in bacterial entry, growth, virulence, enhanced survival and stress conditions within the host. Antigens will be down-selected based on their recognition (and cytokine production) by NTM-infected human peripheral blood mononuclear cells (PBMCs). In addition, the NTM candidate antigens will be screened using a search for homology to >1,700 clinical NTM isolates. We plan to characterize the responses of immune-enhancing agents by evaluating innate immune cytokine production with varying doses of these agents in combination with the NTM antigens, as well as testing their ability to kill the mycobacteria using activated macrophage and dendritic cell-killing assays. Prior to testing the vaccine candidates in our challenge models, we plan to include a mycobacterial growth inhibition assay (MGIA) that will be adapted to NTM.
By including innovative approaches and models, this will project will accelerate new NTM treatment regimens. This R01 project will develop a prophylactic anti-mycobacterial treatment candidate against clinical NTM isolates, including both M.abs and Mycobacterium avium (M.av) and will offer an alternative strategy to drug treatment in at-risk individuals.
Schistosomiasis
A Phase 1, Randomized, Placebo Controlled Trial to Evaluate the Safety, Tolerability, and Immunogenicity of the Sm-p80 + GLA-SE (SchistoShield®) Vaccine in Healthy Adults
DMID Protocol Number: 18-0018
DMID Funding Mechanism: Vaccine and Treatment Evaluation Units
The study is a Phase 1, randomized, blinded, placebo controlled, dose escalation clinical trial to evaluate the safety, tolerability, and immunogenicity of the Sm-p80 + GLA-SE vaccine candidate in 48 healthy adults. Four treatment groups will be evaluated: A) 100 μg Sm-p80 (unadjuvanted), B) 10 μg Sm-p80 + 5 μg GLA-SE, C) 30 μg Sm-p80 + 5 μg GLA-SE, and D) 100 μg Sm-p80 + 5 μg GLA-SE. Each group will include nine subjects randomized to receive the Sm-p80 product and three subjects to receive placebo and all subjects will receive three intramuscular (IM) injections of 0.5 mL of the designated study product, on Days 1, 29, and 57 (28 days apart).
