CAR T Cell Therapy Offers Hope Against DIPG
Published
Featured Researchers

 Gavin is thriving in kindergarten. Top image: Dr. Nick Vitanza, known as "Dr. Tanza" to his patient Gavin Nielsen.
Gavin is thriving in kindergarten. Top image: Dr. Nick Vitanza, known as "Dr. Tanza" to his patient Gavin Nielsen.In 2021, when Ashlee and Nate Nielsen first learned their 2-year-old, Gavin, had an incurable brain tumor, the answer to “How long?” was devastating. Children with diffuse intrinsic pontine glioma (DIPG) generally survive for eight to 11 months after diagnosis.
Ashlee remembers telling her mom, “He’s never going to get to go to kindergarten.”
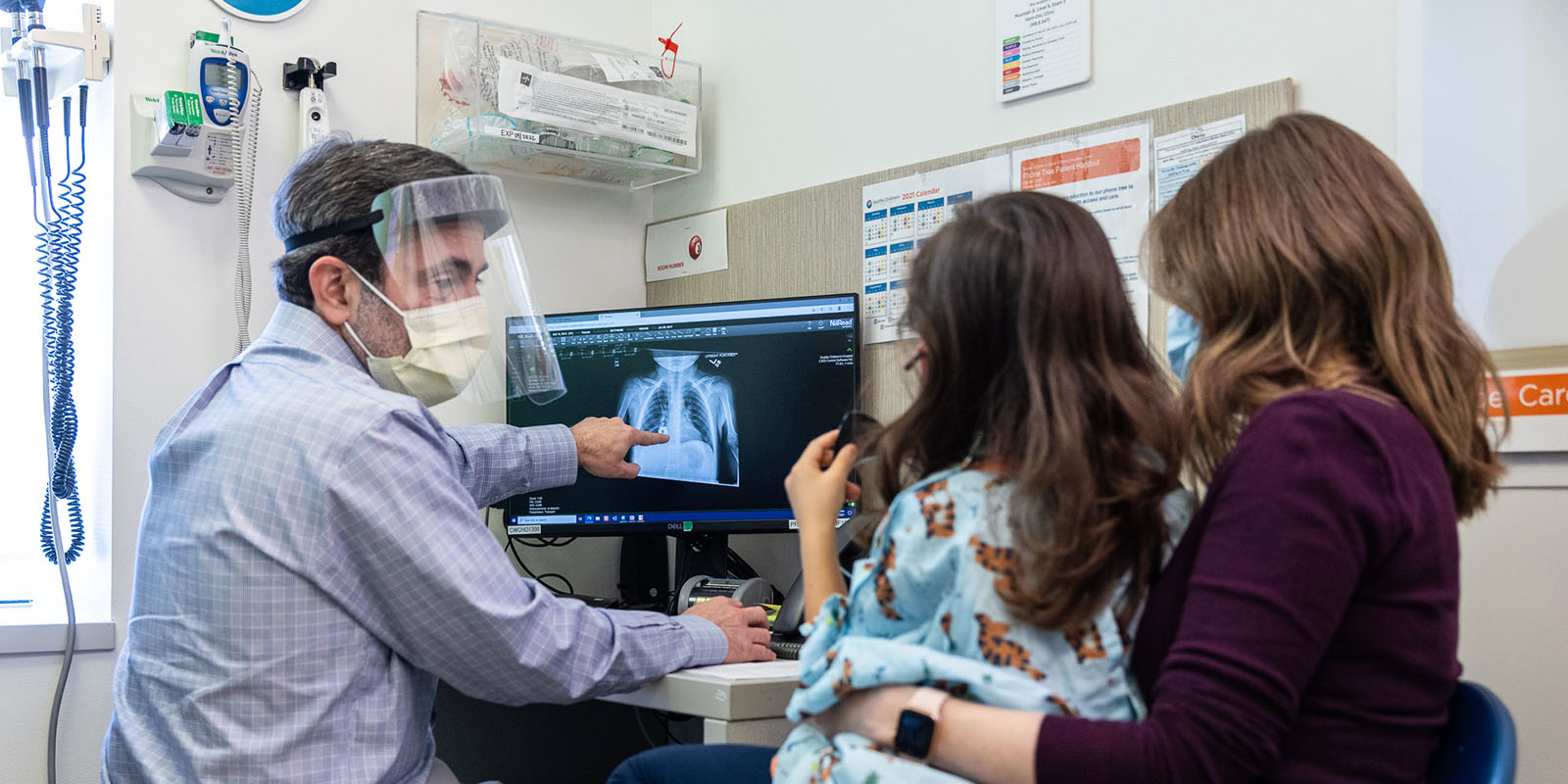
Fortunately for Gavin, Nicholas Vitanza, MD, a pediatric neuro-oncologist and scientist at Seattle Children’s Research Institute’s Ben Towne Center for Childhood Cancer and Blood Disorders Research, was launching BrainChild-03, a clinical trial testing whether chimeric antigen receptor T cells (CAR T cells) could safely be used against these deadly tumors. Gavin was the youngest kid with DIPG to join the study.
CAR T cell therapy uses the body’s own immune system to help fight cancer. Immune cells called T cells are taken from the patient’s blood and engineered in the lab to have receptors (CARs) added on their surfaces to recognize cancer cells. Gavin’s engineered cells were put back intracranially — dosed directly into a fluid cavity within his brain — where the T cells had the new ability to target B7-H3, a protein found on cancer cells.
CAR T cell therapy has shown success in treating several blood cancers; in some cases, producing remissions lasting more than a decade. But using CAR T cells to treat solid tumors is more challenging due to limitations in entering the tumor, and in the case of brain tumors, penetrating the blood-brain barrier, which is why Seattle Children’s dosed CAR T cells directly into the brain.
‘Encouraging’ Study Results
This week, Dr. Vitanza and colleagues published the first-in-human clinical trial results in the journal Nature Medicine, showing that B7-H3-targeting CAR T cells delivered intracranially were well tolerated in 21 treated children and young adults with DIPG. The CAR T cells also may have improved their survival; however, Dr. Vitanza cautions that phase 1 trials best determine safety and larger trials are required to confirm if treatments are beneficial.
“When we began this trial, children with DIPG had never received intracranial CAR T cells so showing the tolerability of these CAR T cells and having some patients live multiple years is very encouraging,” Dr. Vitanza said.
Living His Best Life
 Gavin plays T-ball, soccer and basketball year-round.
Gavin plays T-ball, soccer and basketball year-round.Gavin is now a thriving kindergartener who, at four years from his diagnosis, has quadrupled his life expectancy.
Dr. Vitanza says Gavin is “clinically doing amazing with no obvious signs of disease.” To his grateful family, that looks like Gavin living his best life: Eagerly heading to school each day, learning to read and write, acting out Minecraft and Pokemon with his pals at recess and playing sports on the weekends.
Every two to three weeks, the Nielsens bring Gavin to Seattle Children’s for a fresh dose of CAR T cells. In Gavin’s words, “I get a poke in my head and then I feel sick because my fighters are fighting my lump.”
The “fighters” don’t keep him down for long. “He gets the infusion and usually he's not feeling well for about 12 to 14 hours afterwards. And then he's back to baseline the next morning,” Nate said.
Gavin experiences headaches, nausea and vomiting after his infusions. Sometimes, he’s raring to go after a few hours: “He'd basically be running out of the clinic, and we're like, ‘Dude, no, come back, let's take it easy,’” Ashlee said.
Gavin recently received his 85th dose of CAR T cells, likely the most of any child or adult in the world.
“His MRI shows only a small amount of remaining abnormality,” Dr. Vitanza said. “And when you are interacting with him, it would be very hard to know that he was diagnosed with an aggressive brain tumor four years ago.
“Gavin’s family has said they don't want to stop treatment. We don’t know the right number of doses, when to stop or any of those things, so since he's been doing well, we're just continuing,” he said.
The BrainChild-03 study tested escalating doses of CAR T cells. Gavin was the first to start the study in the second dose group, which was 25 million cells. He now receives 50 million cells at a time.
“There have been a lot of firsts for us. It’s very scary because there’s no comparison. And it has remained that way because we are still in uncharted territory almost four years later as he’s now been on the trial the longest,” Ashlee said.
‘You Wouldn’t Even Know He’s Sick’
Gavin’s parents say that seeing their son flourish is worth the uncertainty. “Not only is he going to school, but he's doing soccer, T-ball and basketball year-round. If you saw him out running on those fields, you would have no way to differentiate him from another kid who's not going through treatment,” Nate said.
Ashlee agrees: “I can’t tell you the number of times people have seen Gavin and said, ‘You wouldn’t even know he’s sick.’ It happens all the time.”
“I don't have the words to express what Children’s commitment to research means. Without it, things would look so different for us, for Gavin. Many kids, not only from all over the United States, but from all over the world, don't have this access. Especially for the DIPG community, Seattle Children's is one of the top places to be. We're so lucky it’s so close for us.”
– Ashlee Nielsen, Gavin’s mom
 Ashlee, Nate, Gavin and Aurora Nielsen
Ashlee, Nate, Gavin and Aurora Nielsen“People were very concerned about whether intracranial CAR T cell therapy would even be possible for children with DIPG and concerned about how many doses they could tolerate,” Dr. Vitanza said. “Now, having a group of kids who collectively have had hundreds of doses is really encouraging because it's created a foundation that we can build on for years with the goal of improving these therapies and improving the lives of more children.”
Although several children in the study might also have benefited from the CAR T cells, not all children responded as well or lived as long as Gavin. Three patients — all of whom began the CAR T treatment prior to disease progression — are still alive, with two of them still receiving CAR T cells years beyond their diagnosis. Dr. Vitanza is eager to improve the odds.
“We still have a lot of work to do in finding out why this therapy wasn’t beneficial for some patients and how to make the benefit even longer lasting for the children who respond,” Dr. Vitanza said.
“The fact that some of these children are having a benefit is just really amazing to see. We have learned a lot about how to run these trials and how this can be done safely, and that gives us the opportunity to do a lot of building — building better CAR T cells and then finding things that they partner with,” he said.
Dr. Vitanza wrote the BrainChild-03 study and served as the founding principal investigator and study chair under the guidance of Julie Park, MD (now chair of St. Jude Children’s Research Hospital’s Department of Oncology), and Michael Jensen, MD, who previously led Seattle Children’s Therapeutics, an on-site cell manufacturing facility which produced the CAR T cell products used in the trial and which oversaw the study’s clinical conduct. Now, Rebecca Ronsley, MD, FRCPC, has taken over as the principal investigator.
Dr. Jensen is the founder and chief scientific officer of a Seattle Children’s spinout biotechnology company, BrainChild Bio, Inc., launched in 2023 to build on Seattle Children’s Therapeutics’ work and to accelerate the advancement of CAR T cell therapies. The company contracts with Seattle Children’s to use its cell therapy manufacturing facilities. BrainChild Bio plans to begin a multi-center Phase 2 pivotal clinical trial by the end of this year based on BrainChild-03 study data.
The launch of the BrainChild-03 study was supported by the Avery Huffman DIPG Foundation, Liv Like a Unicorn, Live Gray’s Way, Love for Lucy, McKenna Claire Foundation, the Pediatric Brain Tumor Research Fund Guild of Seattle Children’s, Team Cozzi Foundation, Unravel Pediatric Cancer, the DIPG/DMG Research Funding Alliance, the We Love You Connie Foundation and St. Baldrick’s Stand Up to Cancer Dream Team Translational Cancer Research Grants (Stand Up to Cancer is a division of the Entertainment Industry Foundation and research grants are administered by the American Association for Cancer Research).
— Colleen Steelquist
Learn More
Featured Researchers
Focus Areas
Related Centers & Programs
PedAL Initiative Makes Progress Against Pediatric High-Risk Leukemia
Published
Seattle Children’s Dr. Todd Cooper leads the worldwide PedAL Initiative and the drive for better options for kids with blood cancers.

Seattle Children’s Study Finds ‘Home Run’ Survival Improvement for Kids With Most Common Form of Leukemia, Changing Care
Published
Seattle Children’s Dr. Todd Cooper leads the worldwide PedAL Initiative and the drive for better options for kids with blood cancers.


