Publication Q&A: Ndufs4 Inactivation in Glutamatergic Neurons Reveals Swallow-Breathing Discoordination in a Leigh Syndrome Model
Published
Featured Researchers
Article Summary
Seattle Children’s neuroscience researchers believe better understanding of Leigh syndrome swallow-breathing discoordination will pave the way for treatments.
Leigh syndrome is a rare genetic condition that affects the central nervous system. A newborn with Leigh syndrome seems healthy at birth. Over time, cells in the nervous system break down or degenerate. Symptoms tend to appear between 3 months and 2 years old. The condition is fatal, with most children passing away by age 3.
Leigh syndrome is a type of mitochondrial disease. Mitochondria are the body’s energy factories, found in all cells except red blood cells. Mitochondria convert the energy in fatty acids and glucose into a substance that energizes cells. Mitochondrial diseases occur when mitochondria don’t function as they should. Leigh syndrome damages or destroys cells in the nervous system that provide energy to the brain, nerves and spinal cord.
About 45% of patients with Leigh syndrome have some type of feeding and swallowing disorder. These impairments contribute to a high risk of developing respiratory infections (aspiration pneumonia), respiratory failure or a failure to thrive, all common causes of death in Leigh syndrome.
 Dr. Alyssa Huff
Dr. Alyssa HuffLed by Alyssa Huff, PhD, a postdoctoral researcher in the Ramirez Lab, and Franck Kalume, PhD, a neuroscientist and principal investigator, both in the Norcliffe Foundation Center for Integrative Brain Research, this study aimed to better understand how mitochondrial dysfunction disrupts swallow-breathing neurocircuitry, with the ultimate goal of finding an effective treatment.
The researchers hypothesized that a Leigh syndrome-causing mutation of the NDUFS4 gene disrupts not only normal breathing but also swallowing. Dr. Huff, who has extensively studied dysfunction in excitatory neurons of swallow and breathing control regions in the brain, hypothesized that exposing the preclinical model to chronic hypoxia — an approach proven in other studies to protect against oxidative stress and neurodegeneration in Leigh syndrome models — might prevent swallow and breathing abnormalities, stabilize body weight and increase lifespan.
Children’s contributing authors: Alyssa Huff, PhD (first author), Luiz Oliveira, PhD, Jan Marino (Nino) Ramirez, PhD, and Franck Kalume, PhD (corresponding author).
This research was supported by grants from the National Institutes of Health.
Read this article in the journal Experimental Neurology (published March 2025).
Is this Leigh syndrome research a first in any way?
Yes, this is the only study in which swallow and its coordination with breathing has been investigated in a preclinical model of Leigh syndrome. While the use of chronic hypoxia (11% oxygen) has been previously shown to reverse lesions in the brain, maintain body weight and increase longevity in a genetic model of Leigh syndrome (Jain et al 2016), our study is the first to show this treatment can restore/prevent loss of functional behaviors necessary for safe feeding practices, such as swallowing and breathing.
What are the potential impacts of this mitochondrial disease research?
We have documented in this preclinical model of Leigh Syndrome that the act of swallowing disrupts breathing for 30 breaths before returning to a normal rhythm. This offers insight into why feeding sessions for children with Leigh Syndrome are so difficult and exhausting. Coordinating swallowing and breathing is essential for life. Without this, feeding is impaired, pneumonia can occur, quality of life declines and failure to thrive occurs, all of which increases the stress and impact on the child and their caregivers.
This study identified critical areas in the brainstem that drive the development of these clinical manifestations of Leigh syndrome. These specific brain regions can serve as target sites for therapy for this condition.
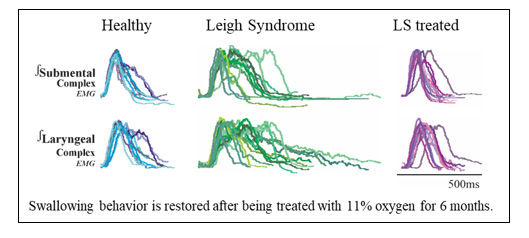 Swallowing behavior was restored through 11% oxygen treatment for six months.
Swallowing behavior was restored through 11% oxygen treatment for six months.
The reason why 11% oxygen (chronic hypoxia) cures or restores swallowing and breathing, as well as maintains body weight and increases life expectancy, is unknown. The Ramirez Lab is investigating why this treatment works so it can be replaced with a drug. Once that drug is produced, we have the preclinical model ready to test this drug. In parallel, the Kalume Lab is developing a genetic approach to counter the many manifestations of this syndrome.
What are the next steps for this swallowing-breathing research?
Using this preclinical model, we are developing methods to observe drinking, swallowing and breathing in a more natural state. This will allow us to investigate all aspects of feeding sessions, not just swallowing. The experimental methods used in this study are also being translated to develop a preclinical model for Rett syndrome [a rare, severe and progressive disorder that impacts a child’s ability to speak, walk, eat or breathe easily].


