A Novel Protein-Degradation Platform With Applications in Cancer and Brain Disorders
Developing drugs that can cross the blood-brain barrier to capture and eliminate pathogenic proteins
Technology Overview
 Dr. James M. Olson
Dr. James M. Olson Dr. Zachary Crook
Dr. Zachary Crook
Drug resistance limits the effectiveness of many small-molecule and antibody-based therapeutics that block or inhibit pathogenic proteins. Mechanisms of resistance include upregulation or mutation of the disease-related protein. As a therapeutic strategy, targeted degradation of pathogenic proteins is less susceptible to resistance mechanisms: Completely removing a protein prevents all signals or interactions that lead to disease outcomes.
Pediatric neuro-oncologist James M. Olson, MD, PhD, and research scientist Zachary Crook, PhD, developed a novel platform that can be adapted to specifically target disease-associated proteins for removal by natural cellular degradation processes. They named this platform CYpHER (CatalYtic pH-dependent Endolysosomal delivery with Recycling).
CYpHER comprises a bispecific protein with one domain that binds a target protein — which can be soluble or membrane associated — in a pH-dependent manner. The other domain binds to a transferrin receptor (TfR) on a cell membrane. When CYpHER-bound TfR is taken into the cell, it brings in the entire complex, including the bound target protein, for delivery to an acidic endolysosome. In this low-pH environment, the target pathogenic protein is released and degraded. The TfR returns to the cell surface, still carrying the now-empty CYpHER molecule, which is ready to capture another target protein and repeat the process (see figure).
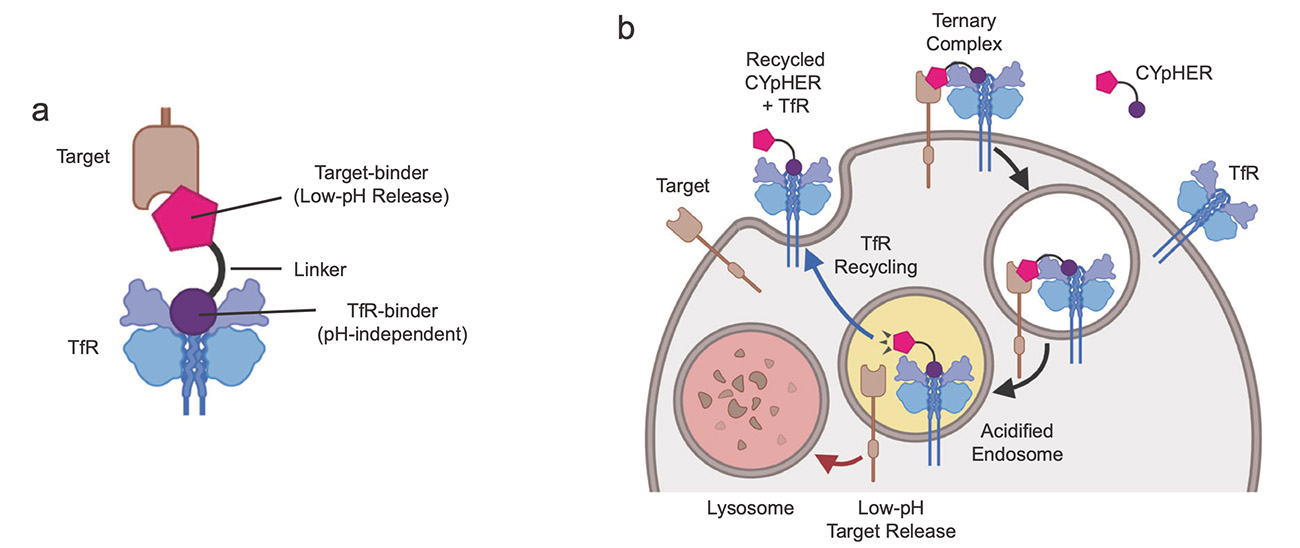
Figure. a, CYpHER molecule with target binding domain linked to domain that binds transferrin receptors (TfR). b. The recycling TfR complex repeatedly delivers target proteins to endolysosomes for degradation. Source: Crook et al., Nat Commun. 2024;15(1):8731.
CYpHER molecules are protein-based molecules, which gives them high target specificity. Their modular design enables their use on virtually any extracellular target, and the TfR-binding capabilities facilitate crossing the blood-brain barrier. Binding domains that can be used in CYpHER molecules include cystine-dense peptides that are stable miniproteins that can quickly be adjusted for properties including affinity for their ligands. These molecules are amenable to computational design and Dr. Crook trained in these methods as a Washington Research Foundation Institute for Protein Design Fellow with Nobel Laureate David Baker, PhD.
Proof-of-concept studies demonstrated in vitro and in vivo effectiveness of CYpHER systems for removing the cancer-promoting factor PD-L1 and the epidermal growth factor receptor (EGFR) that is often mutated in cancers. Moreover, TfR is overexpressed in and required for the growth of many cancers, which could naturally increase the effectiveness of CYpHER-based therapeutics for cancer. Furthermore, TfR is substantially overexpressed on the blood vessels of the brain, making it an ideal carrier for delivering drugs to the central nervous system.
Drs. Olson and Crook are collaborating with Invent postdoctoral scholar Akinsola Oyelakin, PhD, and neuroscientist Nino Ramirez, PhD, to develop CYpHER as a therapy for early schizophrenia. High complement 4 (C4) levels are a risk factor for schizophrenia development. A CYpHER system that decreases C4 levels in individuals diagnosed with early schizophrenia is in preclinical development. Dr. Crook is also leading research on anti-inflammatory CYpHER molecules to treat conditions including central nervous system inflammation and cancer treatment-associated neurotoxicity.
Drs. Olson and Crook have extensive experience working with industry partners and Dr. Olson has cofounded multiple biotech companies. The researchers are interested in partnerships to advance the development of the CYpHER platform and explore its clinical applications in diseases of interest to industry collaborators.
Stage of Development
- Preclinical in vitro
- Preclinical in vivo
Partnering Opportunities
- Collaborative research and development
- Sponsored research agreement
- Consultation agreement
- Animal model access
- Licensing agreements
Publications
Crook ZR, Sevilla GP, Young P … Olson JM, et al. CYpHER: catalytic extracellular targeted protein degradation with high potency and durable effect. Nat Commun. 2024;15(1):8731.
Crook ZR, Girard EJ, Sevilla GP … Olson JM. Ex silico engineering of cystine-dense peptides yielding a potent bispecific T cell engager. Sci Transl Med. 2022;14(645):eabn0402.
Crook ZR, Girard E, Sevilla GP … Olson JM. A TfR-binding cystine-dense peptide promotes blood-brain barrier penetration of bioactive molecules. J Mol Biol. 2020;432(14):3989-4009.
Crook ZR, Nairn NW, Olson JM. Miniproteins as a powerful modality in drug development. Trends Biochem Sci. 2020;45(4):332-346.
Crook ZR, Sevilla GP, Friend D … Baker D … Olson JM, et al. Mammalian display screening of diverse cystine-dense peptides for difficult to drug targets. Nat Commun. 2017;8(1):2244.
Learn More
