Seattle-PAP
A Lifesaving Innovation for Infant Respiratory Distress
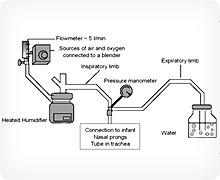
The key components of a Bubble-CPAP system.
Faced with the global need for affordable, safe, easy-to-use and easy-to-maintain respiratory support technology, the researchers in the NeoRest team have invented a device that can provide critical respiratory support to infants who may otherwise receive little or no assistance. The device, called Seattle Children's Positive Airway Pressure (Seattle-PAP) device, is similar to, but fundamentally improves upon, a commonly used respiratory support technology called Bubble Continuous Positive Airway Pressure (B-CPAP).

A Seattle-PAP device concept model.
B-CPAP supports spontaneous breathing by delivering a continuous, pressurized gas flow to an infant's airway. The gas is usually humidified air, enriched with oxygen, and is delivered to the infant's nose though a breathing circuit and nasal prongs. The pressure of the delivered gas is controlled by simply adjusting the depth of a partially submerged tube attached to the end of the infant's breathing circuit. B-CPAP may provide additional benefits over conventional nasal CPAP systems because as gas exits the submerged tube it forms bubbles that create small airway pressure oscillations. These oscillations are transmitted to the patient's lungs and are thought to improve gas exchange, enhance lung recruitment and reduce the work of breathing.
The team's Seattle-PAP device improves upon conventional B-CPAP by providing an equivalent mean airway pressure accompanied by oscillations that may reduce the likelihood an infant will need to receive mechanical ventilation. The key difference of the Seattle-PAP device is that the expiratory tube is placed in the water at a 135° angle; conventional B-CPAP tubes are placed in the water at 0° (straight down). It is this change in angle that more consistently produces the range of oscillations thought to improve lung function and make it easier for an infant to breathe.
Importantly for resource-limited countries' healthcare settings, Seattle-PAP can provide respiratory support for a fraction of the cost of conventional ventilators. It is also far easier to operate and maintain. Once the device is available commercially, Seattle-PAP will allow healthcare providers to focus on an infant's clinical needs rather than managing the bedside technology.
With critical help from the Bill and Melinda Gates Foundation, the NeoRest Team finalized the Seattle-PAP device design, identified manufacturers to produce the device and launched clinical trials. If these trials are successful, Seattle-PAP could fundamentally transform respiratory care and bring a lifesaving advance to infants worldwide.
