Current Research at the Gust Lab
Reseach in the Gust Lab integrates cutting-edge preclinical models, biomarker discovery, and detailed investigation of cellular and molecular processes to uncover how CAR T cell therapies interact with the brain—advancing safer, more effective immunotherapies for cancer patients.
CAR T Cell Trafficking Into Gliomas
![]() This project develops an advanced animal model of chimeric antigen receptor (CAR) T cell cancer immunotherapy for malignant brain tumors, which has been challenging in the clinical setting because of limited ability of the CAR T cells to travel to the tumors and survive in the hostile microenvironment.
This project develops an advanced animal model of chimeric antigen receptor (CAR) T cell cancer immunotherapy for malignant brain tumors, which has been challenging in the clinical setting because of limited ability of the CAR T cells to travel to the tumors and survive in the hostile microenvironment.
We will use an immunocompetent transgenic animal model to induce brain-intrinsic tumors via viral delivery of oncogenes into brain progenitor cells, and image the tumor growth and CAR T cell infiltration into the tumor using two-photon microscopy via a cranial window.
This model enables us to study of molecular mechanisms of CAR T cell targeting against brain tumors by manipulating T cell guidance cues, T cell signal recognition, and adhesion molecule activation.
Clinical Biomarkers of CAR T Cell Neurotoxicity
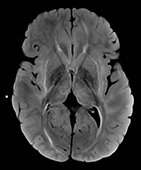 The overarching goal of this study is to make cancer therapies less toxic to children’s developing brains. Specifically, we propose to develop direct measurements of neurologic injury via blood and cerebrospinal fluid biomarkers in pediatric leukemia patients. The biomarker measurements will then be used to determine which therapies cause the most brain and nerve injury. In a subgroup of patients, we will use brain imaging and neurocognitive testing to measure whether changes in neurologic injury biomarkers are correlated with decreases in cognitive performance. This information will allow us to integrate simple and validated measurements of neurologic injury into future clinical trials of chemotherapy and radiation regimens in a wide variety of pediatric cancers. Taking neurocognitive development into consideration as an essential outcome will allow us to change the paradigm of cancer treatment toward a focus on living a healthy, independent, and productive future life after beating cancer.
The overarching goal of this study is to make cancer therapies less toxic to children’s developing brains. Specifically, we propose to develop direct measurements of neurologic injury via blood and cerebrospinal fluid biomarkers in pediatric leukemia patients. The biomarker measurements will then be used to determine which therapies cause the most brain and nerve injury. In a subgroup of patients, we will use brain imaging and neurocognitive testing to measure whether changes in neurologic injury biomarkers are correlated with decreases in cognitive performance. This information will allow us to integrate simple and validated measurements of neurologic injury into future clinical trials of chemotherapy and radiation regimens in a wide variety of pediatric cancers. Taking neurocognitive development into consideration as an essential outcome will allow us to change the paradigm of cancer treatment toward a focus on living a healthy, independent, and productive future life after beating cancer.
Pediatric leukemia treatment has made impressive advances over the past few decades, and now >80% of children are cured of the disease with standard chemotherapy regimens. However, treatments are multiple years long and grueling both emotionally and physically. Dangerous toxicities are common. Injuries to the brain are an especially important consideration, as they can be irreversible and lead to decreased ability to achieve a child’s full academic, social, and professional potential. Chemotherapy can also frequently cause peripheral neuropathy, which can be painful and debilitating and cause lifelong problems with balance and strength.
The biomarkers we will measure are well validated readouts of brain cell injury in a variety of neuroinflammatory and neurodegenerative disorders, which makes them suitable for our study. Neurofilament light chain (NFL) is a protein that is part of nerve cells in the brain and the body, and injury releases this into the blood and the cerebrospinal fluid. The second biomarker is glial fibrillary acidic protein (GFAP), which is an important component of the glial support cells in the brain. In previous work, we have found that children and young adults with relapsed leukemia have extremely high levels of NFL and GFAP, often higher than what is seen in adults with severe brain problems such as dementia. This led us to ask how this injury develops over the time of treatment, and whether there are specific interventions that cause a rise in these markers.
Mechanism of CAR T Cell Neurotoxicity
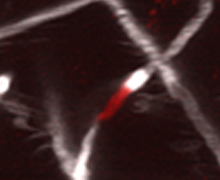 This project studies the mechanism of neurologic toxicity in chimeric antigen receptor (CAR) T cell therapy. CAR T cells are genetically modified, patient derived T cells that use the CAR to recognize and destroy malignant target cells. Although CAR T therapy has shown impressive results against leukemia and lymphoma, approximately 30-40% of patients experience neurologic side effects in the first month after receiving CD19-targeted CAR T cells. This includes cognitive disturbances, seizures, and in rare cases fatal cerebral edema. Systemic cytokine release syndrome after CAR T cell infusion is a well-established risk factor for neurotoxicity, but the connection between systemic inflammation and brain dysfunction is poorly understood.
This project studies the mechanism of neurologic toxicity in chimeric antigen receptor (CAR) T cell therapy. CAR T cells are genetically modified, patient derived T cells that use the CAR to recognize and destroy malignant target cells. Although CAR T therapy has shown impressive results against leukemia and lymphoma, approximately 30-40% of patients experience neurologic side effects in the first month after receiving CD19-targeted CAR T cells. This includes cognitive disturbances, seizures, and in rare cases fatal cerebral edema. Systemic cytokine release syndrome after CAR T cell infusion is a well-established risk factor for neurotoxicity, but the connection between systemic inflammation and brain dysfunction is poorly understood.
To study the mechanisms of neurotoxicity, we have developed an immunocompetent animal model. After treatment with high dose CD19-directed murine CAR T cells, the models develop motor and balance difficulties, as well as brain microhemorrhages. Surprisingly, we found that >10% of cortical capillaries are obstructed by white blood cells during neurotoxicity. This was accompanied by capillary remodeling and decreased vessel coverage by pericytes.
We are now working to uncover the molecular mechanisms that cause white blood cells to plug capillaries during neurotoxicity. We use in vivo two-photon imaging in animal models to determine which cell types cause the capillary plugging – the animal’s own or the transferred CAR T cells? We will then measure how CAR T cell treatment changes the expression of adhesion molecules in brain capillary endothelial cells and in leukocytes, and test whether blockade of these adhesion interactions can prevent capillary plugging and neurotoxicity.
