Cherry Lab
The Cherry Lab investigates how the visual system develops, and how genetic variations contribute to blindness and other visual disorders. Our ultimate goal is to develop new therapeutic strategies to treat these disorders.
Unlocking the non-coding genome in vision and disease
More than 250 genes are mutated in human visual disorders. However, 30-50% of cases of inherited visual disorders cannot be explained by mutations in protein-coding genes alone. This strongly suggests that mutations within non-coding regions of the genome contribute to human vision and inherited visual disorders.
Promoters and enhancers are major functional components of the non-coding genome that work together to regulate the precise timing, cell-type-specificity and physiologically-appropriate levels of expression of their target genes. Individual studies have shown that mutations within enhancers of retinal disease genes can cause human visual disorders. However, it has been impossible to systematically identify and interpret most of these mutations because the genomic locations and functions of human retinal enhancers are largely uncharacterized.
The Cherry Lab utilizes high-throughput DNA and RNA sequencing technologies to identify and characterize human and mouse retinal enhancers to elucidate the transcriptional regulation of retinal development and function. This powerful dataset has already allowed us to discover novel disease-associated enhancer variants in patients with inherited retinal disorders.
Understanding how enhancers contribute to disease
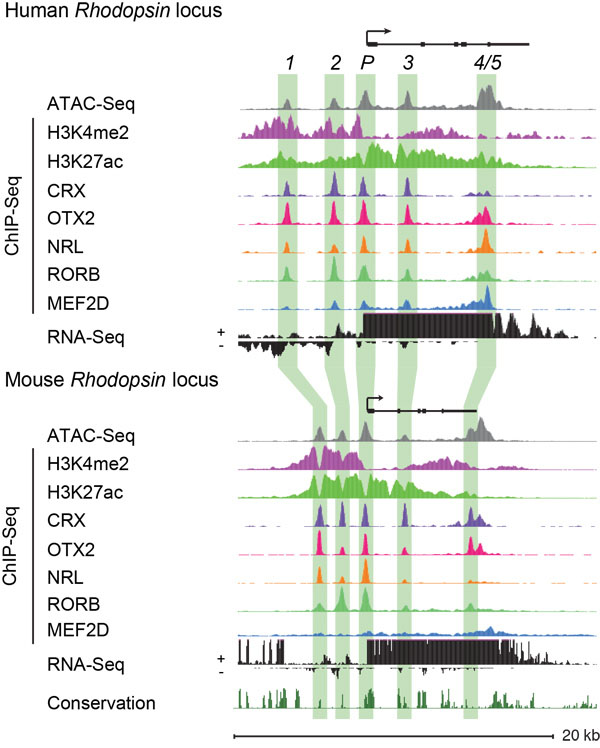 We are now tackling the next major hurdle in understanding the significance of enhancers in retinal function and disease, by developing the ability to manipulate enhancer function in the native context of the human genome. Most genes, including those that are essential for retinal function, have multiple enhancers that work together to regulate gene expression. However, exactly how these enhancers work together is poorly understood.
We are now tackling the next major hurdle in understanding the significance of enhancers in retinal function and disease, by developing the ability to manipulate enhancer function in the native context of the human genome. Most genes, including those that are essential for retinal function, have multiple enhancers that work together to regulate gene expression. However, exactly how these enhancers work together is poorly understood.
Our goal is to determine the contribution of enhancers to the regulation of critical retinal disease genes using methods to test the role of these elements in the context of the endogenous genome. This project’s aims include:
- Establish a human in vitro platform to model retinal disease-associated enhancer function.
- Determine the requirement of enhancers for retinal disease-associated gene regulation in vivo.
Pursuing cures for visual disorders
Our lab is also working to “rewire” genetic switches, with the goal of correcting the genetic problems that drive visual disorders. We are pursuing ways to instruct stem cells or skin cells to become healthy retinal cells. Our vision is to someday use these cells to replace faulty cells and cure visual disorders in children and adults.
Data Resources
Partnership Opportunities

Timothy Cherry, PhD
Timothy Cherry, PhD, is an assistant professor at the University of Washington School of Medicine’s Department of Pediatrics and Division of Genetic Medicine, and a principal investigator at Seattle Children’s Research Institute’s Center for Developmental Biology and Regenerative Medicine.
-

Abbi Engel
Abbi Engel joined the Cherry Lab as lab manager/research scientist in March 2022. Her role is be assisting with iPSC stem cell models of disease and assisting in day-to-day lab operations and administration. She completed her PhD via the NIH GPP (graduate partnership program) with Georgetown University and the National Institutes of Health in 2010, followed by a postdoc in cancer immunology and natural products with a collaborative project between Bastyr University (Kenmore, Washington) and the University of Washington.
-

Santiago Fregoso
Santiago Fregoso is a postdoctoral fellow who joined the Cherry Lab in September 2021. His work is focused on uncovering the role of microRNA in the genetic programs that regulate specification and differentiation of neural progenitors in the developing brain. Santiago received his PhD from the University of Colorado Anschutz Medical Campus in 2019.
-
Ashi Jain
-

Leah VandenBosch
Research Scientist III
Leah completed her PhD in molecular and cellular biology at the University of Washington in the lab of Dr. Tom Reh in 2019. Her doctoral studies focused on epigenetic regulation of the development and potential regeneration of the mammalian retina. For her postdoctoral research, she is lending her skills to the exploration of non-coding genetic variation and characterization of disease associated variants in the human retina through Machine Learning and Massively Parallel Reporter Assays (MPRA). Hobbies: Outside of the lab, Leah enjoys many creative outlets such as baking, crocheting, music, and painting. Fun fact: Leah shares her last name with a genus of fern, Vandenboschia.
