Publication Q&A: Impaired Capillary-Venous Drainage Contributes To Gliosis and Demyelination in White Matter During Aging
Published
Featured Researchers
Abstract
Utilizing innovative high-resolution imaging, this study is the first to link age-related cognitive decline and reduced cerebral blood flow to structural and functional degradation of Principal Cortical Venules, establishing deep venous networks as a novel therapeutic target for age-related neurological diseases.
Latest research from the Shih Lab, at Seattle Children’s Research Institute’s Center for Developmental Biology and Regenerative Medicine and Norcliffe Foundation Center for Integrative Brain Research, published in Nature Neuroscience, suggests that age-related reduced blood flow to the brain, which leads to cognitive decline, may partly result from impaired drainage through deep networks of tiny blood vessels. Improving blood flow could potentially prevent damage to the brain over time.
Read this publication in Nature Neuroscience.
Is this neuroscience research a first in any way?
Our study is the first to apply innovative high-resolution imaging techniques in the brain of a preclinical model to understand age-related changes in blood vessels supporting vulnerable areas of the brain. These experiments revealed previously unrecognized similarities in vascular structure between small-animal models and humans.
We found that sparse venules, termed Principal Cortical Venules, and their complex branching networks, are responsible for draining deep brain tissues known to degrade during aging. Alterations in these venules are responsible for poorer blood flow, leading to brain inflammation and the loss of cerebral white matter essential for brain connectivity.
How can this research lead to better ways to treat, prevent or diagnose cognitive decline and disease?
Improving blood flow to the brain is an essential therapeutic goal for many age-related neurological diseases. Alzheimer’s disease and related dementias are an escalating global health concern in the aging population over the coming decades. One of the earliest changes in Alzheimer’s disease is vascular dysfunction, highlighting an urgent need to understand how degradation of small brain vessels impairs nutrient delivery and drives cognitive decline. However, imaging techniques for the living human brain (MRI) cannot resolve the smallest brain blood vessels. Therefore, high-resolution imaging in small-animal models is essential to understand microvascular changes in disease.
Our work reveals the cerebral venous networks as a potential therapeutic target for treatment. Specific changes include increased inflammation of vascular walls, and contraction and loss pericytes — cells critical for controlling flow of blood through the finest vessels (capillaries). Now we can more precisely tease apart the mechanisms that drive the initiation and progression of these diseases.
Our findings may also be relevant to other neurological conditions affecting younger individuals experiencing neuroinflammation. For example, a prior study showed that inflammation in principle cortical venules may contribute to cerebral white matter damage in patients with multiple sclerosis.
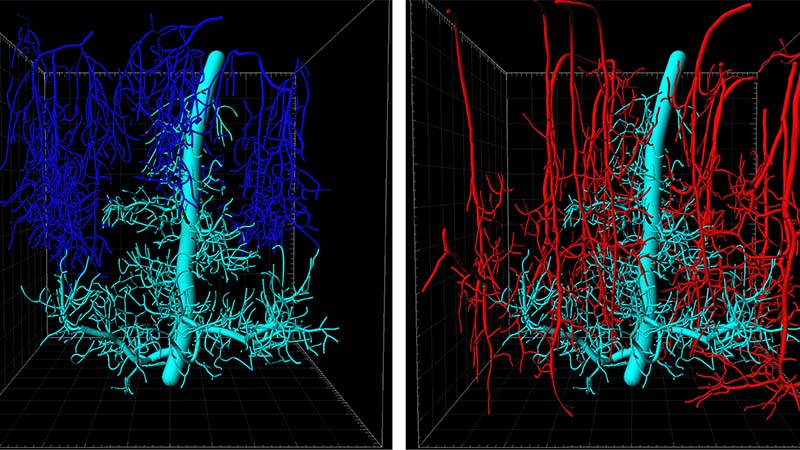
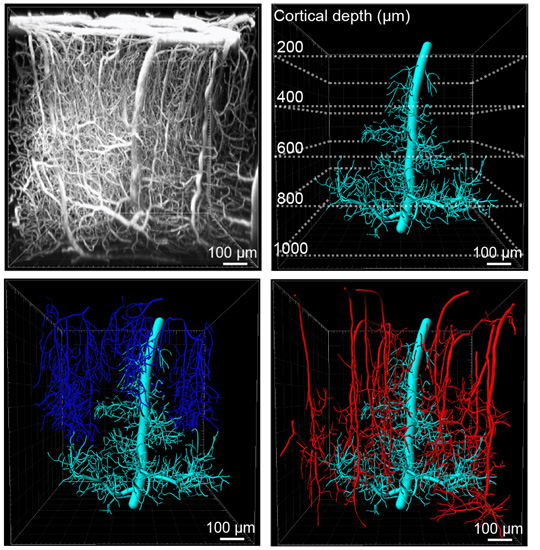 Figure legend: Upper left image: Side view of the microvasculature in the somatosensory cortex showing a 900 x 900 x 1000 μm (x, y, z) volume collected by in vivo deep two photon imaging. Upper right image: 3D reconstruction of a Principal Cortical Venule (PCV) from the volume together with branching capillary networks draining into the PCV (cyan). Dotted lines mark the depth below the brain surface. Lower left image: PCV with all other ascending venules (blue) draining the depicted volume. Lower right image: PCV with all penetrating arterioles (red) supplying blood to the depicted volume.
Figure legend: Upper left image: Side view of the microvasculature in the somatosensory cortex showing a 900 x 900 x 1000 μm (x, y, z) volume collected by in vivo deep two photon imaging. Upper right image: 3D reconstruction of a Principal Cortical Venule (PCV) from the volume together with branching capillary networks draining into the PCV (cyan). Dotted lines mark the depth below the brain surface. Lower left image: PCV with all other ascending venules (blue) draining the depicted volume. Lower right image: PCV with all penetrating arterioles (red) supplying blood to the depicted volume.
What are the next steps and long-term goals for this research?
We are continuing this research in several directions.
First, we will examine the mechanisms that contribute to structural and functional changes of brain vasculature during aging. To do this, we are leveraging our close collaboration with the researchers from the Allen Institute for Brain Science on the use of state-of-the-art spatial transcriptomics approaches to understand early gene expression changes in vascular cells that might contribute to degradation of capillary-venous structure and function.
The second is to test whether existing clinically used therapeutics known to dilate cerebral blood vessels are effective at promoting blood flow through capillaries and venules in older brains.
Third, we hope to understand how reduction of blood flow in deep layers of the brain contributes to white matter degeneration in Alzheimer’s disease. We are investigating deep vascular networks in transgenic models that recapitulate certain aspects of Alzheimer’s disease pathology in humans. Our initial findings indicate that deep capillary-venous networks are affected at disease onset, and may, in fact, be part of the initial mechanism leading to disease progression.
These research directions will help us establish the exact cause-and-effect relationships that lead to vascular dysfunction and identify potential early biomarkers, as well as therapeutic targets that could be utilized for early diagnosis and development of therapies aimed at stopping or slowing down inadequate blood flow to body tissue, during aging and dementia.
How was this work supported at Seattle Children’s and other funding sources?
This study was largely conducted at the Center for Developmental Biology and Regenerative Medicine at Seattle Children's Research Institute, and supported by advanced microscopy and infrastructure within the Center. This paper was also authored by Seattle Children’s researchers Gokce Gurler, MD, PhD, Nicolas Weitermann, Stephanie Bonney, PhD and Maria Sosa. To perform parts of the work we leveraged the services of the Children’s Shared Research Resources Histology and Imaging cores.
This work was supported by grants from the National Institutes of Health’s National Institute of Aging and National Institute of Neurological Disorders and Stroke, and a a pilot award from the Albert Institute for White Matter Research.
Parts of the work have been performed in collaboration with research groups at the Allen institute for Brain Science, Pennsylvania State University and ARTORG Center for Biomedical Engineering Research at Switzerland’s University of Bern.

