Current Research at the DiBlasi Lab
Aerosolized Surfactant Research
Nearly 4 million infants die worldwide yearly, with 1 million dying principally from respiratory insufficiency in low- and middle-income countries (LMICs) due to a lack of respiratory support devices and therapies. The DiBlasi Lab has worked with the Bill and Melinda Gates Foundation and industry partners to study aerosolized surfactants. In an effort to bridge a major gap in lung protection and infant mortality from surfactant deficiency and respiratory distress syndrome (RDS), developing a surfactant aerosol and delivery system would prevent or delay disease progression, dramatically reduce airway and pulmonary injury, extend the functional capabilities of nasal noninvasive support (i.e., CPAP) and prevent intubation in a large fraction of infants that would otherwise receive noninvasive mechanical ventilation, or die from surfactant deficiency where ventilators can’t be accessed.
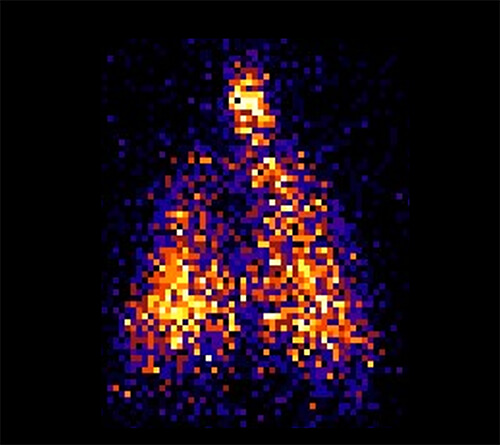
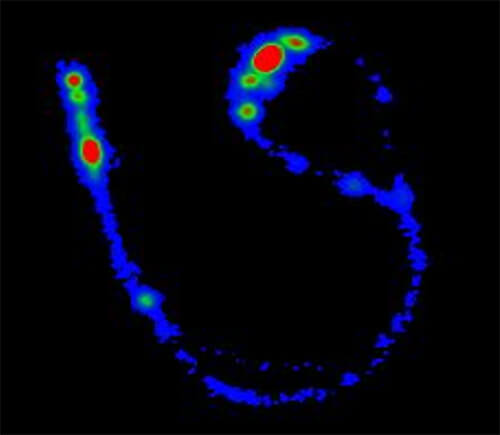
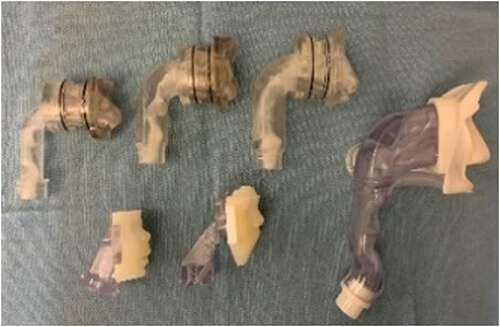
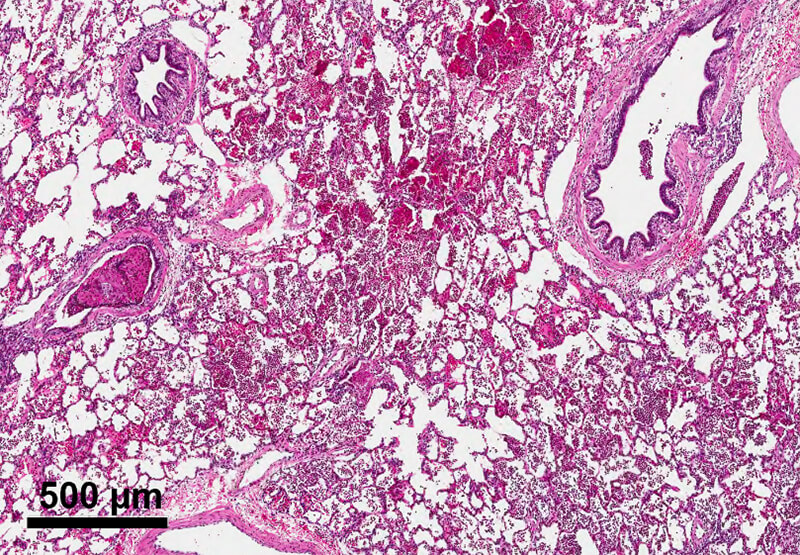
This partnership has led to the development of several lead candidate drug formulations, including synthetic dry powder and liquid surfactant aerosols and breath-synchronized nebulizers that can be safely integrated with nasal CPAP. State-of-the-art research capabilities, like laser diffractometry, are being used to characterize aerosol particle size in the aerosol laboratory. Nebulizer delivery efficiency and safety are evaluated during in vitro experiments to estimate pulmonary medication delivery using realistic high-fidelity breathing simulators and 3D-printed anatomic upper airways. Gamma scintigraphy is used to quantify radioaerosol deposition in the upper airways and lungs and accounts for inadvertent medication losses within the CPAP delivery hoses and interfaces. In vivo studies apply surfactant aerosols in surfactant-deficient lungs to evaluate dose-response effects on gas exchange, lung mechanics, lung injury/inflammation and other physiologic outcomes.
Evaluation of High-Frequency Airway Clearance Devices
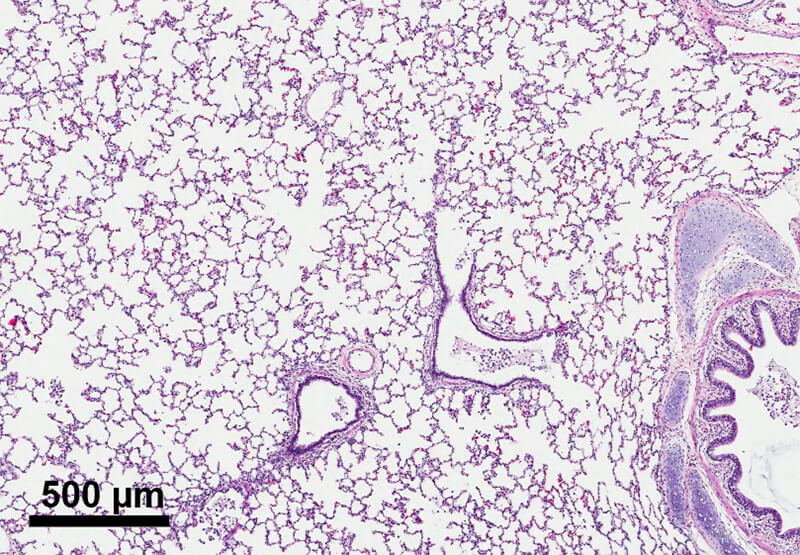
Mucus can accumulate in the airways of children with severe lung infections and impaired cough. The DiBlasi Lab has partnered with industry sponsors to evaluate several novel devices that generate inhaled aerosols combined with high-frequency (300-400/min) flow pulsations, or “mini coughs,” to expand the airways, facilitate secretion removal and improve lung function. The lab has produced an artificial mucus simulant that is applied to realistic airway and lung models to evaluate the effect of mucus transport at different frequencies and pressure settings. Ongoing work is focused on the effectiveness of mucus removal in physiologic models of mucus impaction.
Neonatal and Pediatric EIT Study
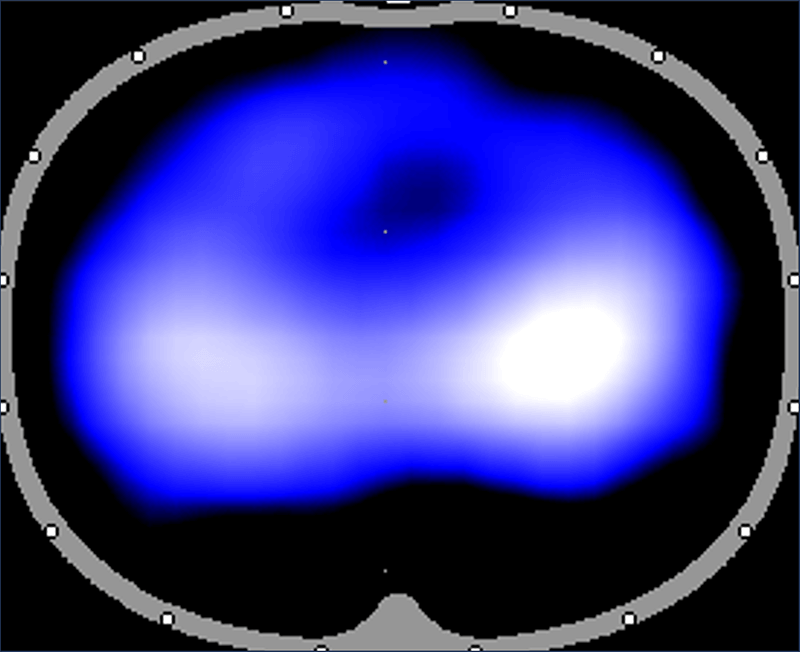
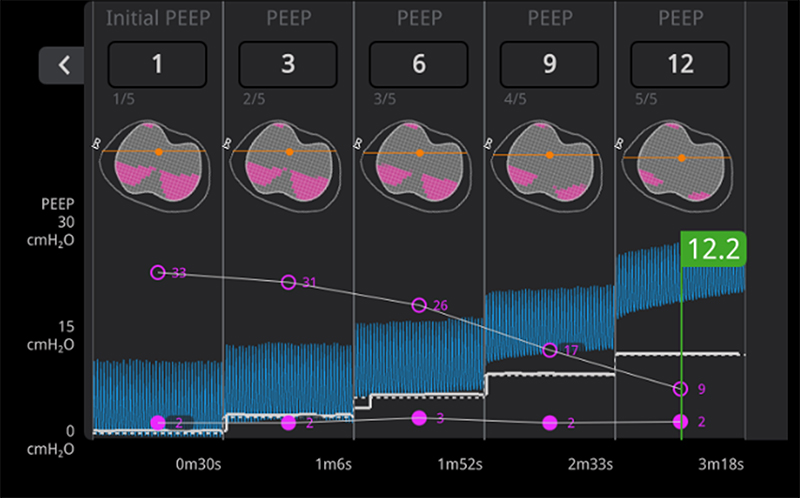
There are very few objective measurements that can enable clinicians to evaluate lung expansion at the bedside. Electrical impedance tomography (EIT) is a novel radiation-free noninvasive imaging tool that analyzes real-time changes in lung volumes with small electrodes to guide bedside management of critically ill mechanically ventilated patients in the intensive care unit (ICU). EIT technology is being designed for neonatal and pediatric patients by Sentec Medical. Sentec has funded the DiBlasi Lab to conduct preclinical research to validate EIT measurements with lung volumes measured by single photon emission tomography (SPECT). These experiments are necessary for FDA approval and widespread availability of this novel technology for future clinical use.