The Immune Mechanisms of Protection Against Mycobacterium tuberculosis Centers (IMPAc-TB) program is an initiative established by NIAID in 2019 to elucidate the immune responses needed to protect against infection with Mycobacterium tuberculosis (M.tb). The program will lead to a better understanding of tuberculosis (TB) immunology, which is critical to guide the design and development of new and improved TB vaccines, and it aligns with the goals of the NIAID Strategic Plan for Tuberculosis Research (see a PDF of the full strategic plan).
Under this initiative, three IMPAc-TB contracts were awarded to the following principal investigators:
- Rhea Coler, MSc, PhD, Seattle Children’s Research Institute: Coler Lab
- Kevin Urdahl, MD, PhD, Seattle Children’s Research Institute: Urdahl Lab
- Sarah Fortune, MD, Harvard University, Henry Boom, MD, Case Western Reserve University, JoAnn Flynn, PhD, University of Pittsburgh: Fortune Lab
Phoenix IMPAc-TB Contract Under Dr. Rhea Coler
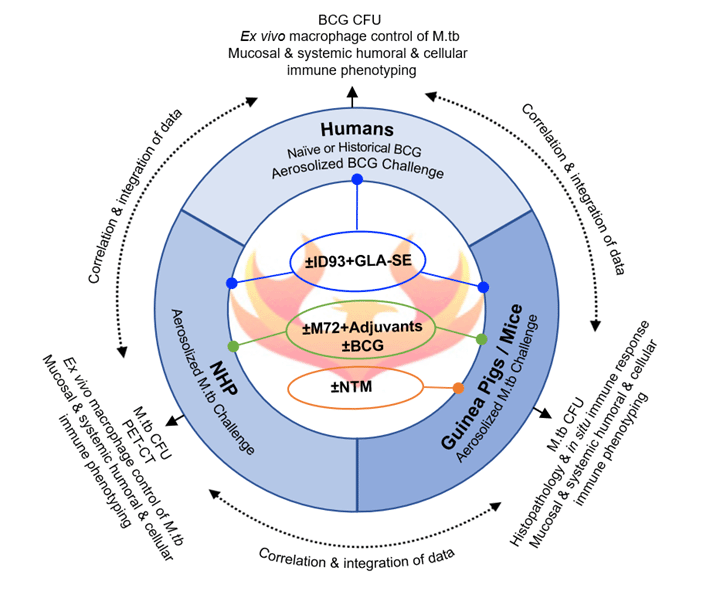
The goal of the Phoenix IMPAc-TB contract is to comprehensively identify the complex immune responses required to prevent M.tb infection or active TB disease by comparing and interrogating the mycobacteria- and vaccine-induced protective immunity in several animal species and in man.
The overarching paradigm of this project is that identification and validation of common protective correlates of immunity against M.tb (a NIAID Category C pathogen) in escalating preclinical animal models of TB, nontuberculous mycobacteria (NTM) exposure, and human challenge experimental medicine clinical studies, will be crucial in the rational design and development of candidate vaccines that generate robust levels of durable, protective immunity against TB. We have assembled a team of researchers from eight academic or translational institutions who are leaders in various fields, including preclinical animal models of TB, human challenge models, clinical and systems immunology, genomics, microbiology, statistical and bioinformatics methodology, nanoparticle formulation, cGMP manufacturing through fill and finish, regulatory, quality control, clinical trials, and development of linked platform technologies and immunology assessments.
Coler Lab Phoenix IMPAc-TB Team at Seattle Children’s Research Institute
 Hazem Abdelaal
Hazem Abdelaal
Fellow PhD
 Sabiriin Abdi
Sabiriin Abdi
Student Helper
 Sasha (Larsen) Akins
Sasha (Larsen) Akins
Research Scientist IV
 Susan Baldwin
Susan Baldwin
Research Scientist, Senior
 Bryan Berube
Bryan Berube
Research Scientist IV
 Bryan Brown
Bryan Brown
Acting Assistant Professor
 Matthew Harband
Matthew Harband
Research Technician I
 Rakhi Harne
Rakhi Harne
Fellow PhD
 Suhavi Kaur
Suhavi Kaur
Research Technician II
 Dana Miller
Dana Miller
Research Technician I
 Deepika Nag
Deepika Nag
Fellow PhD
 Tiffany Pecor-Korman
Tiffany Pecor-Korman
Research Associate III
 Thomas Smytheman
Thomas Smytheman
Research Technician II
 Alison Wald
Alison Wald
Scientific Project Manager I
 Brittany Williams
Brittany Williams
Graduate Student
 Zhiyi Zhu
Zhiyi Zhu
Research Scientist II
Phoenix IMPAc-TB Partners
- Seattle Children’s Research Institute
- Fred Hutchinson Cancer Research Center: Andrew Fiore-Gartland
- United Kingdom Health Security Agency: Sally Sharpe
- La Jolla Institute of Immunology: Cecilia Lindestam Arlehamn
- Denver Research Institute: Edward Chan
- Colorado State University: Marcela Henao-Tamayo, Brenden Podell
- University of Oxford: Helen McShane
- Ragon Institute: Ryan McNamara

