Cardiac Microvascular Remodeling in Heart Disease
A complex vascular network within myocardial tissue maintains a high input of oxygen and nutrients to support cardiomyocyte contraction. As stress on the heart causes myocardial tissue remodeling, cardiac endothelial cells respond by regenerating a functional microvascular plexus in the remodeled tissue. The ability for cardiac endothelial cells to reactivate developmental mechanisms and mature into a functional vascular plexus underlies dynamic cardiac resilience to injury.
We are investigating what mechanisms are critical in cardiac neovascularization; which processes driving microvascular maturation are disrupted in heart disease; and how we can use therapeutic approaches to promote vascular plexus maturation in the heart.
Somatic Mutations in Myocardial Tissue
Each cell’s DNA acquires somatic mutations from proliferation or exposure to mutagens. These mutations, which can be single nucleotide mutations or chromosomal abnormalities, can have drastic effects on cell functions and promote cardiovascular disease.
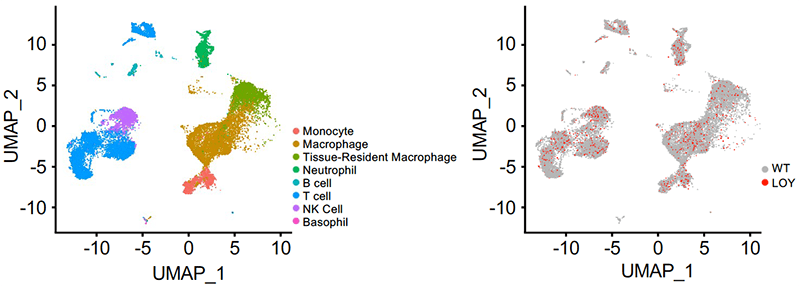
The most well-studied mutations are in hematopoietic cells. One such mutation occurs in men – blood cells will lose their Y chromosome with age, termed “Loss-of-Y” (LOY). This is the most prevalent known somatic mutation and previous studies have shown a causal connection with non-ischemic heart failure.
We are investigating how LOY in blood cells promotes pathogenic myocardial remodeling through intrinsic leukocyte functions and extrinsic effects on cardiac interstitial cells. Furthermore, we are also investigating general clonal expansion of cardiac cells during myocardial remodeling and underlying effects of somatic mutations.
Cardio-oncology
It is now understood that cancer treatments can have cardiovascular complications in some patients. Acute cardiomyopathy secondary to chemotherapy is a risk for many cancer patients, but treatments can also cause long-term damage resulting in early-onset heart failure. This is particularly devastating for pediatric oncology patients who can experience heart failure symptoms before 30 years old.
We are investigating how some chemotherapies can cause damage to cardiac interstitial cells and promote pathogenic remodeling, leading to extremely early-onset age-related cardiomyopathy, with the aim to identify strategies to increase cellular resilience to chemotherapies.