James T Bennett, MD, PhD

Specialties
- Children's Title: Attending Physician
- Academic Title: Associate Professor, Department of Pediatrics, University of Washington School of Medicine
- Research Center: Center for Developmental Biology and Regenerative Medicine
- On Staff Since: August 2012
"Applying novel diagnostic approaches for specific genetic diagnosis in children with vascular anomalies and mosaic disorders "
-
Biography
James Bennett, MD, PhD, is an associate professor in the Department of Pediatrics, Division Genetic Medicine, and and adjunct associate professor in the Department of Laboratory Medicine and Pathology. His clinical and research focus is on vascular anomalies and other genetic disorders caused by post-zygotic mosaicism.
Board Certification(s)
Medical Genetics, Clinical Molecular Genetics
Medical Genetics, Clinical Genetics (M.D.)
Education
New York University School of Medicine, New York, NY
Residency
University of Washington, Seattle, WA
Clinical Interests
Vascular anomalies
Mosaic Disorders
Application of Genetic Testing to rare pediatric diseases
Research Description
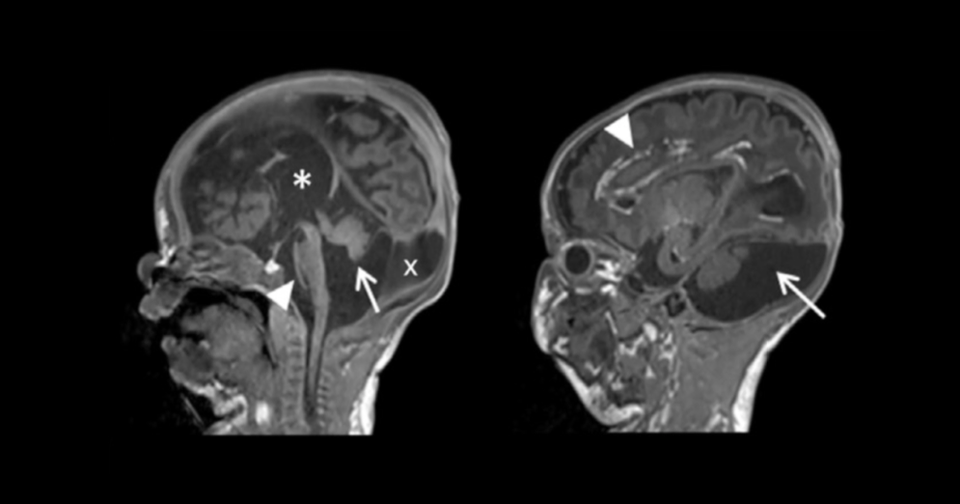
Almost all genetic sequencing is performed from blood or saliva derived DNA, which implicitly assumes that the genomes of blood cells are the same as every other cell in the body. Because errors occur in DNA replication with every division, all individuals carry genetic variants that are not present in every cell in the body. This has major implications for diagnosis of children with genetic disorders, since pathogenic mutations that are absent, or present at low levels in the blood will not be detected by current standard methods.
To address this, the Bennett lab develops methods for accurate detection of these "hidden" low-level mosaic variants, with a major focus on vascular anomalies. We are also interested in measuring the "normal" rate and distribution of somatic variation in individuals across their lifespan.
Research Focus Area
Somatic Mosaicism, vascular anomalies
-
Related Resources
-
Family thankful for revolutionary gene sequencing technology at Seattle Children’s
KIRO radio news story, 6/1/2016
-
James Bennett, MD, PhD Researcher Profile
View the Seattle Children's Research Institute profile of James Bennett including their publications and grants.
-
The Bennett Lab at Seattle Children's Research Institute investigates the contribution of "hidden" post-zygotic mutations on human development and birth defects, with a focus on vascular malformations.
-
How mouse kidneys led Dr. Jimmy Bennett to career in genetics
Puget Sound Business Journal, 6/23/2016
-
Dr. Bennett's publications on MyBibliography
View a list of Dr. Bennett's publications on MyBibliography.
-
SpotSeq: A New Non-Invasive Test to Diagnose Genetic ‘Hotspots’ in Pediatric Vascular Malformations
Brotman Baty Institute News, 12/1/2022
-
The Boy Missing an Entire Type of Brain Cell
The Atlantic, 4/11/2019
-
Scientists join NIH study of genomic variation among cells
UW Medicine News Release, May 11, 2023
-
-
Patient Testimonials
-
Awards and Honors
Loading...Award Name Award Description Awarded By Award Date {{ award.name }} {{ award.description }} {{ award.organization }} {{ award.displayDate }} No Awards and Honors found for James T Bennett, MD, PhD
-
Publications
Loading...No Publications found for James T Bennett, MD, PhD
-
Presentations
Loading...Presentation Title Event Location Date {{ presentation.title }} {{ presentation.presentedAt }} {{ presentation.location }} {{ presentation.displayDate }} No Presentations found for James T Bennett, MD, PhD
-
Research Funding
Loading...Grant Title Grantor Amount Award Date {{ funding.title }} {{ funding.grantedBy }} {{ funding.amount }} {{ funding.displayDate }} No Research Funding found for James T Bennett, MD, PhD
-
Clinical Trials and Research Studies
Loading...{{ item.st }}
No clinical trials found for James T Bennett, MD, PhD.