Altered hyaluronan deposition by bronchial epithelial cells contributes to inflammation during respiratory syncytial virus infection
Lower respiratory tract infections commonly caused by respiratory syncytial virus (RSV) are a leading cause of hospitalizations and mortality worldwide in young children. Despite many years of study, the mainstay of treatment remains supportive care and currently no RSV specific treatment or vaccine is available.
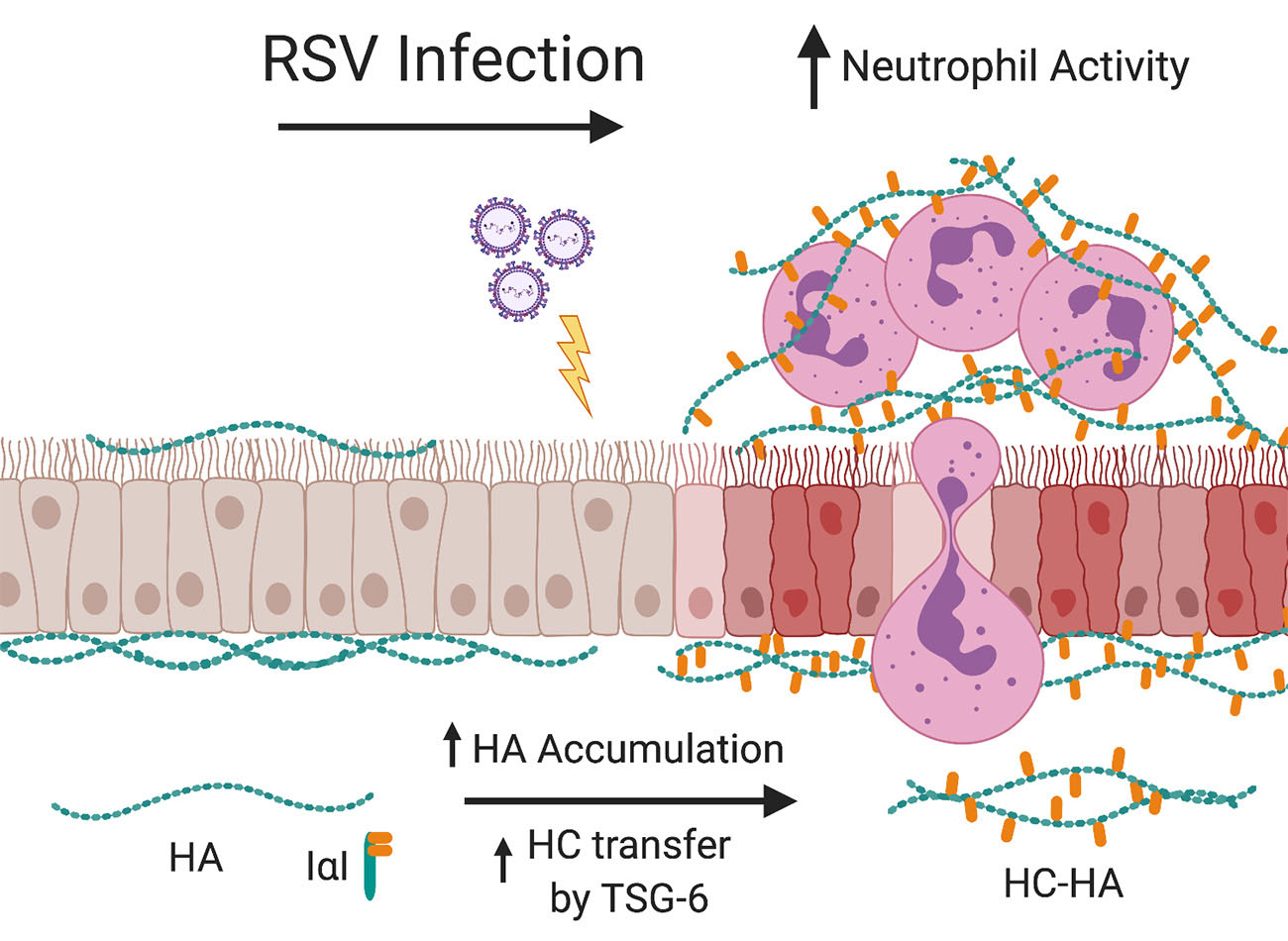
The cells that line the airways, otherwise known as bronchial epithelial cells (BECs), are the primary target for RSV infection. Infection of the BECs with RSV leads to subsequent BEC damage, obstruction of the lower airways with cellular debris and mucous, and the establishment of airway inflammation by recruitment of immune cells such as neutrophils into the peribronchial space. Recent studies from our laboratory have demonstrated that RSV infection of stromal cells such as lung fibroblasts leads to the establishment of an extracellular matrix (ECM) that is enriched with hyaluronan (HA) which promotes the accumulation and activation of leukocytes in ex vivo cell culture models.
Furthermore, we found that the increased HA accumulation following RSV infection was more closely associated with the fibroblast cell layer and displayed greater modification with heavy chains (HC) which has been described in other studies to enhance the ECM’s ability to become sticky for inflammatory cells and promotes the inflammatory response in the lung.
The formation of HC-HA is the result of the enzymatic activity of tumor necrosis factor stimulated gene 6 (TSG-6), which is not normally expressed in healthy lung tissue, but is induced in response to injury. In our previous work with lung fibroblasts, we have shown that TSG-6 is upregulated during RSV infection and blocking its induction with siRNA decreased the amount of HC-HA that was formed and decreased the accumulation of leukocytes.
Despite being the primary target of RSV infection, no studies exist that have evaluated the effects of RSV infection on BEC production of HA or TSG-6 induced HC-HA formation despite the important role that HC-HA plays in promoting lung inflammation.
Our laboratory both has expertise in the characterization of HA matrices and access to primary human BECs from well-characterized pediatric donors placing our group in a unique position to evaluate the contribution of BEC derived HC-HA to the inflammatory response following RSV infection.
We hypothesize that RSV infection of BECs leads to increased production and accumulation of HA which will in turn promote the accumulation and activation of neutrophils in an ex vivo human cell culture model system.
Additionally, we will test the hypothesis that RSV infection of BECs will induce the expression of TSG-6 thereby promoting the formation of HC-HA and further drive the accumulation and activation of neutrophils in our model system.
We will pharmacologically block the formation of HC-HA enriched ECMs and also block the induction of TSG-6 during RSV infection in order to establish whether the increased accumulation of the neutrophils is HC-HA dependent.
If true, characterizing the importance of HC-HA produced by BECs may lead to novel targets for therapeutic intervention during acute RSV infections and may be applicable to other respiratory viruses.